| x | x | ||||
 |
|||||
| BACTERIOLOGY | IMMUNOLOGY | MYCOLOGY | PARASITOLOGY | VIROLOGY | |
|
|
|
||||
|
|
|||||
|
|
|||||
|
Let us know what you think |
|||||
|
TEST YOUR KNOWLEDGE |
|||||
|
Acute gastroenteritis (mainly, infectious diarrheas) is second only to
cardiovascular disease as a cause of death worldwide. In the U.S., acute
gastroenteritis is second only to viral respiratory disease as a cause of acute
illness, prompting an estimated 73 million physician consultations each year.
Most episodes are self-limited but infectious diarrhea and various foodborne
illnesses account annually for more than 5,000 deaths. Other intraabdominal
infections are relatively common and present dilemmas in diagnosis and
management. |
|||||
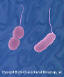
 Figure 1
Figure 1Helicobacter pylori - Gram-negative, spiral to pleomorphic, spiral rod prokaryote. © Dennis Kunkel Microscopy, Inc. Used with permission |
Helicobacter pylori H. pylori (figure 1) is a spiral-shaped, microaerophilic gram-negative rod that attaches to epithelial cells in the stomach, especially in the antrum, where it resides in the mucous layer. H. pylori damages mucous-secreting cells and also promotes an acute and chronic inflammatory response that may lead to a sequence of events: acute gastritis followed by chronic superficial gastritis, then atrophic gastritis, intestinal metaplasia, dysplasia, and adenocarcinoma. The stomach infected with H. pylori also develops lymphoid follicles (lymphoid tissue is not normally present in the stomach), which can evolve into a low-grade malignancy known as MALT lymphoma. Why some persons develop these various manifestations of H. pylori infections while others do not remains largely unclear. H. pylori colonizes at least one-half of the world’s population, with high colonization rates in developing countries where the infection is usually acquired during childhood. About 35% of persons in the U.S. are colonized and, as the frequency of colonization is about 0.5% per year, about 50% of persons > 60 years of age in the U.S. and other developed countries may be infected. Fecal-oral transmission occurs, and lower prevalence rates correlate with higher standards of living and sanitation. H. pylori is associated with about 70% to 90% of duodenal ulcers, about 70% of gastric ulcers, and with about 60% to 80% of gastric carcinomas. There is evidence that the prevalence of H. pylori in the U.S. has been declining, and it is suggested that this may explain, at least in part, the declining incidence of peptic ulcer disease and adenocarcinoma of the stomach. However, the incidence of reflux esophagitis, Barrett’s esophagus, and adenocarcinoma of the esophagus in the U.S. has been increasing, leading to the provocative suggestion that certain strains of H. pylori may be protective against the serious consequences of gastroesophageal reflux disease (GERD).
|
||||
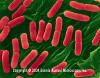 Figure 2
Figure 2E. coli (0157:H7) hemorrhagic type. Gram-negative, enteric, facultatively anaerobic, rod prokaryote. Potentially fatal to humans, contracted when contaminated meat is cooked inadequately. © Dennis Kunkel Microscopy, Inc. Used with permission
|
Food PoisoningCentralized food processing and distribution combined with the growing trend toward meals away from home make food poisoning an increasingly important problem in the U.S. and elsewhere. About 5000 outbreaks of food-borne disease are reported to the CDC each year, but the magnitude of the problem is thought to be far greater. By various estimates, 6 million to 81 million cases of food poisoning occur in the U.S. each year, causing 323,000 hospitalizations and 5,000 deaths with expenditures exceeding $5 billion. Symptoms range from mild facial flushing (Chinese restaurant syndrome) or gastroenteritis to life-threatening paralysis or colitis. Emerging problems include the hemolytic-uremic syndrome caused by Shiga toxin-producing strains of E. coli (notably E. coli 0157:H7 (figure 2) but also others) and increasing drug resistance among Salmonella, Shigella (figure 3), and other relatively common bacteria. Here we will review briefly the major syndromes of food poisoning. Bacterial food poisoning can result from one of 4 mechanisms:
Toxin production and/or tissue invasion is characteristic of infection due to Vibrio parahaemolyticus (figure 4 and 5) and Yersinia enterocolitica (figure 6), which thus seem to cause disease by more than one mechanism. Some microorganisms, for example Bacillus cereus, cause disease by more than one mechanism. Salmonella species are the most frequently reported cause of food-borne outbreaks and are sometimes associated with severe disease. Shigella species, the classic agents of bacillary dysentery, currently cause < 2% of outbreaks in the U.S., while Campylobacter jejuni, although responsible for > 200,000 cases of diarrhea each year, causes <1% of reported food-borne outbreaks. Shiga toxin-producing E. coli strains assume importance disproportionate to their frequency because of the severe complications (discussed below). Listeria monocytogenes causes occasional outbreaks of food-borne illness in the United States, which can result in life threatening disease especially in young children and among the elderly. Brucella species cause occasional outbreaks of disease (brucellosis) in the U.S. and are usually acquired from cheese made from unpasteurized milk, raw milk, or raw meat. Parasites associated with outbreaks of food-borne disease in the U.S. in recent years include Cyclospora cayetanensis (cyclosporiasis) and Cryptosporidium parvum (cryptosporidiosis). Listeria monocytogenes has been associated with outbreaks traced to dairy products. The syndromes of food poisoning vary according to the organism, the organ(s) affected and the time of onset after ingestion:
Staphylococcal food poisoning and short-incubation B. cereus food poisoning are characterized by vomiting and crampy abdominal pain. Diarrhea occurs in about one-third of cases. A later onset of crampy abdominal pain with diarrhea is characteristic of Clostridium perfringens and long-incubation B. cereus food poisoning, but these same symptoms can be caused by many other enteric pathogens.
|
||||
Infectious Diarrhea: OverviewInfectious diarrhea is the second most common cause of death worldwide and the leading cause of death in early childhood. In the U.S., infectious diarrhea causes death mainly in older and debilitated persons. However, disastrous consequences can occur in previously healthy persons. Pathogen-associated complications from infectious diarrhea include mycotic aneurysm of the aorta with Salmonella species, the hemolytic-uremic syndrome and renal failure with Shiga toxin-producing strains of E. coli, and Guillain-Barré syndrome with Campylobacter jejuni. The clinician’s primary task is to discern which patients need further investigation including stool cultures, which need empiric antimicrobial therapy, and which need only "tincture of time". According to various estimates, there are > 200 million episodes of diarrhea in the U.S. each year (by one estimate, 1.4 episodes per person), resulting in > 73 million physician consultations, 28 million office visits, 1.8 million hospitalizations, and 5,000 deaths. Answers to a series of questions usually determine the optimum approach to diagnosis and therapy. When a patient presents with diarrhea as the chief complaint, important questions include the following:
|
|||||
|
Shiga toxin-producing E. coli (E. coli 0157:H7 and Other Serotypes) Strains of E. coli that produce Shiga toxin, also known as enterohemorrhagic E. coli strains, were recognized in the U.S. in 1982 and now cause an estimated 20,000 cases of diarrheal disease each year. Although uncommon in primary care (<1% of all cases of infectious diarrhea), these strains can cause severe hemorrhagic colitis, the hemolytic-uremic syndrome, and thrombotic thrombocytopenic purpura. E. coli 0157:H7 differs from most E. coli strains by its production of one or more Shiga toxins and by its inability to ferment sorbitol. Other serotypes of E. coli sometimes produce Shiga toxins, but these are uncommon in the U.S. These E. coli strains adhere tightly to mucosal cells, especially in the ascending and transverse colon. The toxin molecule damages mucosal cells and also enters the systemic circulation, where it causes vascular and endothelial damage that can lead to capillary leak syndrome, thrombosis, hemolysis, and renal failure. Most human infections have been transmitted by beef, and are thought to result from fecal contamination of meat during slaughter. Undercooked hamburger meat has been famously associated with E. coli 0157:H7 outbreaks. It has been estimated that a hamburger purchased at a fast food restaurant in the U.S. contains meat from as many as 1000 cows. Outbreaks have also been associated with the ingestion of unchlorinated drinking water, unpasteurized apple cider, alfalfa sprouts, leaf lettuce, mesclun lettuce, radish sprouts, milk, and other foods and beverages. New standards for food processing have been introduced in the U.S. with the aim of reducing the prevalence of this pathogen. However, the infectious dose is low, possibly as low as 100 bacteria. Therefore, prevention is difficult and person-to-person spread may occur. After an incubation period of 3 to 4 days (range 1 to 8 days), patients classically present with crampy abdominal pain and bloody stools. The pain is often severe and out of proportion to the findings on physical examination of the abdomen. About one third of patients have fever, which characteristically follows the appearance of blood in the stool. Some persons infected with E. coli 0157:H7 remain asymptomatic, and others experience crampy abdominal pain with little or no diarrhea. Most patients, however, have bloody diarrhea caused by hemorrhagic colitis. Data from large series suggest that up to 90% of patients experience bloody diarrhea at some point during the illness, with about 60% of patients having blood in their stools at the time of presentation. Severe abdominal pain prior to the onset of fever often suggests acute appendicitis, while pain combined with bloody stools suggests inflammatory colitis. |
|||||
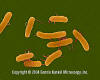 Figure . Salmonella - rod prokaryote (dividing); note the flagella. Causes salmonellosis (food poisoning).
(x 20,800) ©
Dennis Kunkel Microscopy, Inc.
Used with permission
Figure . Salmonella - rod prokaryote (dividing); note the flagella. Causes salmonellosis (food poisoning).
(x 20,800) ©
Dennis Kunkel Microscopy, Inc.
Used with permission
|
Salmonella species other than S. typhi (Salmonellosis) Gastroenteritis caused by non-typhoidal Salmonella strains is extremely common in the U.S., with an estimated 0.8 to 3.7 million cases each year. Many cases, and probably the vast majority of sporadic cases, go unrecognized. Outbreaks are usually associated with food products. Between 1985 and 1994, Salmonella enteritidis was identified with 582 outbreaks that accounted for 28,058 cases of disease, 2,290 hospitalizations, and 70 deaths. Salmonellae are aerobic gram-negative rods. Recent studies showing high levels of DNA similarity among Salmonella isolates have led to the reclassification of all clinically important Salmonellae into a single species, Salmonella choleraesuis. There are 7 subgroups of Salmonella choleraesuis, and more than 2300 serovars are recognized based on 3 major antigens (the somatic O antigen, the surface Vi antigen, and the flagellar H antigens). Most clinical laboratories continue to report isolates by their familiar names; for example, Salmonella typhimurium rather than Salmonella choleraesuis serotype typhimurium. Although S. typhi and S. paratyphi colonize only humans, non-typhoidal Salmonella strains are widespread throughout the animal kingdom. Human disease is usually associated with food products, most commonly poultry and eggs. Infection of poultry flocks is widespread. Ingestion of uncooked or lightly cooked eggs is therefore a risk factor for disease. However, outbreaks and cases have been linked to numerous foods including tomatoes, alfalfa sprouts, cantaloupe, and freshly squeezed orange juice. Some patients with salmonellosis give a history of keeping exotic pets, especially reptiles, of which up to 90% harbor Salmonella organisms. Salmonella infection is frequently asymptomatic. There is no reliable serologic test, and a single negative stool culture does not exclude the possibility that a food handler might be an intermittent shedder of Salmonella organisms. Given both the wide distribution of Salmonella in foodstuffs and the frequency of asymptomatic Salmonella carriage, it is difficult to envision how any restaurant might prevent the occasional case of Salmonella transmission despite emphasis on hygienic practices. Salmonella infection is a risk of everyday life, especially for persons who dine out frequently. Salmonella infection nearly always arises by ingestion of the bacteria. Gastric acidity is the first line of host defense, since Salmonella survives poorly if at all at the normal gastric pH (< 1.5). On the basis of previous data, it has been accepted that ingestion of >105 Salmonella organisms is necessary to cause disease. However, more recent data indicate that a lower inoculum (<103 organisms) can cause disease. Because Salmonella survives well at pH values of 4.0 or higher, persons with atrophic gastritis (which is common among the elderly) or who are taking gastric pH-raising medications (antacids, H2-blockers, or proton pump inhibitors) are at increased risk of salmonellosis. Containment of Salmonella infection depends on an intact T-lymphocyte system including macrophage function. Persons at risk of serious consequences of Salmonella infection include (1) persons with impaired T-cell function because of lymphoproliferative disorders, other malignancies, HIV disease, or immunosuppressive medication; and (2) persons with disorders that cause “macrophage blockade” such as hemoglobinopathies (notably, sickle cell disease), bartonellosis, malaria, schistosomiasis, and disseminated histoplasmosis. The 5 recognized syndromes of salmonellosis are gastroenteritis, enteric fever (discussed separately, below), bacteremia and endovascular infection, localized metastatic infections, and the asymptomatic carrier state. Gastroenteritis due to non-typhoidal Salmonella usually presents as nausea, vomiting, and diarrhea 6 to 48 hours after the ingestion of contaminated food or water. A high inoculum of Salmonella correlates with increased severity and duration of the illness. The stools are usually loose, of moderate volume, and without blood. Occasionally the disease can present as classic “small bowel diarrhea” with large volume, watery stools or as classic “large bowel diarrhea” with small volume stools accompanied by tenesmus. Fever, chills, nausea, vomiting, and abdominal cramps are common. Occasionally, right lower quadrant pain suggesting acute appendicitis dominates the clinical picture (pseudoappendicitis). Bacteremia is documented, when sought, in up to 4% of immunocompetent persons with Salmonella gastroenteritis. Unrecognized episodes of transient bacteremia are probably common. More prolonged bacteremia correlates with immunosuppression. Multiple positive blood cultures for Salmonella should raise the possibility of intravascular infection including mycotic aneurysm or endocarditis. Localized metastatic infection occurs in up to 5% to 10% of patients. The major syndromes are as follows:
The asymptomatic carrier state develops in 0.2% to 0.6% of persons with non-typhoidal salmonellosis, and is more common in women and in persons with abnormalities of the biliary tract. The long-term carrier state is defined by persistence of Salmonella in stool or urine cultures for > 1 year. |
||||
Shigella species (Shigellosis)Shigella species are the classic agents of dysentery and are highly communicable. Shigellosis remains an important cause of morbidity and mortality in developing countries, mainly in children. At least 19,000 cases occur in the U.S. each year. Shigella are gram-negative bacilli with 4 recognized serogroups: group A (Shigella dysenteriae), group B (Shigella flexneri), group C (Shigella boydii), group D (Shigella sonnei). Within these 4 serogroups are about 40 serotypes, of which Shigella dysenteriae serotype 1 (also known as the Shiga bacillus) causes the most severe disease. Shigella sonnei now accounts for about 60% to 80% of cases of shigellosis in the U.S. Like Salmonella, Shigella can contaminate food and water, causing occasional common source outbreaks. However, person-to-person transmission is the dominant mode of transmission. As few as 10 to 100 Shigella organisms can cause disease; therefore, shigellosis is highly communicable. After exposure to a case of shigellosis in a household, about 40% of persons between and 1 to 4 years of age and about 20% of persons of all ages develop the disease. Outbreaks of shigellosis occur in crowded, closed environments such as nurseries, day care centers, institutions, and cruise ships. Following ingestion, Shigella organisms multiply in the small intestine resulting in concentrations of 107 to 109 bacteria per mL of intestinal fluid. Symptoms commonly result from small intestinal involvement, but the hallmark symptoms of shigellosis—the dysentery syndrome—result from mucosal invasion and toxin production in the colon. Inflammation is severe but relatively superficial, and bacteremia is therefore uncommon. |
|||||
 Figure
Figure Campylobacter jejuni is an enteric, curved-rod prokaryote (bacterium). It is the bacterium that causes campylobacteriosis, one of the most common bacterial causes of diarrheal illness in the United States. It is a relatively fragile bacterium that is easily killed by cold or hot temperatures. Birds are carriers due to their body temperature being just right to host the bacteria. Improper handling of raw poultry or undercooked fowl is usually the source of infection in humans. © Dennis Kunkel Microscopy, Inc. Used with permission |
Campylobacter Species Diarrhea caused by Campylobacter jejuni is one of the most common infectious diseases worldwide. More than 1 million cases occur each year in the U.S., where Campylobacter is isolated from stool cultures more frequently than either Salmonella or Shigella species. Campylobacter jejuni is therefore an important pathogen in primary care. Campylobacter fetus is encountered much less frequently and presents as a bacteremic illness. Campylobacter species are small, comma-shaped or curved gram-negative bacilli, of which C. jejuni and C. fetus are of medical importance. C. jejuni causes an intestinal infection manifested as acute gastroenteritis and colitis. Campylobacter fetus is more likely to cause a systemic infection with bacteremia, meningitis, intravascular infections including endocarditis, and metastatic abscesses. Human disease is predominantly food-borne but can result from direct contact with animals, including household pets (notably, puppies or kittens with diarrhea). Fecal-oral transmission occurs, and men who have sex with men are at increased risk. However, transmission from food handlers appears to be uncommon. Some studies suggest that disease can result from ingestion of as few as 500 bacilli. Like Salmonella, Campylobacter is inhibited by hydrochloric acid in the stomach and might therefore occur more commonly when gastric pH is raised because of gastritis or medications. Campylobacter species invade the intestinal mucosa and also elaborate various extracellular toxins with cytopathic activities. Campylobacter jejuni causes disease throughout the year in the U.S., but sharp peaks of Campylobacter disease occur during the summer and early fall. The disease especially affects children < 1 year of age and persons between 15 and 29 years of age. This pattern in the U.S. contrasts sharply with the pattern in developing countries, where Campylobacter affects persons of all ages and especially during the first 5 years of life. Mishandling of raw poultry and consumption of undercooked poultry are now considered to be the major risk factors to Campylobacter infection. The incubation period of Campylobacter jejuni gastroenteritis is usually between 1 and 7 days (average, 2 to 4 days). In about two thirds of cases, the disease begins with abdominal pain and/or diarrhea. The remaining patients have a prodrome that suggests a flu-like illness (see below). Pain is often severe and can be the predominant symptom. In other patients, fever is the predominant manifestation of the disease. The diarrhea can consist of frequent loose stools, massive watery stools, or grossly bloody stools. Most patients (at least 50% in one study) have 10 or more bowel movements on the worst day of the illness. Gross blood is commonly present in bowel movement during the second and third days of the illness. Mild leukocytosis is often present. |
||||
|
Clostridum difficile and Antibiotic-Associated Colitis Diarrhea is a relatively common complication of antimicrobial therapy and is associated with Clostridium difficile in about 10% to 30% of cases. Pseudomembranous colitis due to C. difficile, the most severe form of antibiotic-associated diarrhea, occurs in about 1 in 10,000 courses of antibiotic therapy in ambulatory patients. C. difficile elaborates two large toxin molecules, an enterotoxin known as toxin A and a cytotoxin known as toxin B. About 60% of adults in the U.S. have serum antibodies to C. difficile, suggesting that asymptomatic colonization is relatively common. Prevalence surveys indicate colonization of the colon by C. difficile in about 3% or less of healthy adults, 2% to 8% of elderly persons in nursing homes, and up to 20% of persons who are hospitalized. Person-to-person transmission of C. difficile occurs, and can be reduced by hand washing before and after patient encounters and by use of disposable gloves (followed by hand washing) during direct contact with patients. Symptoms typically begin 5 to 10 days after a course of antimicrobial therapy, but can start as early as the first day of treatment and as late as 10 weeks after treatment has been discontinued. A common presentation consists of acute onset of watery diarrhea with low-grade fever and abdominal pain. The disease ranges in severity from mild to fulminant. Overall, about 30% to 50% of patients have fever and 20% to 33% have abdominal pain. Despite prominent involvement of the rectosigmoid area in most cases, gross blood is seldom present in the stool.
|
|||||
 Figure . Rotavirus (A double-capsid particle (left), and a single, inner, capsid (right))
©
Dr Linda
Stannard, University of Cape Town, South Africa
Figure . Rotavirus (A double-capsid particle (left), and a single, inner, capsid (right))
©
Dr Linda
Stannard, University of Cape Town, South Africa |
Miscellaneous Gastrointestinal PathogensViruses One of the most common human infections, viral gastroenteritis causes an estimated 3 to 5 billion cases of diarrhea each year, worldwide, with some 5 to 10 million deaths. Rotaviruses Rotaviruses, so-named because they resemble a wheel complete with spokes under the electron microscope (rota is Latin for “wheel”), cause about one-third of all diarrhea-related hospitalizations worldwide, with about 800,000 deaths each year. Nearly all children are infected by age 3 years. In the U.S., diarrhea due to rotaviruses accounts for an estimated 500,000 physician visits, 50,000 hospitalizations, and 20 to 40 deaths, at an annual cost > $1 billion. Of the 3 recognized groups of rotaviruses (A, B, and C) are recognized, group A accounts for most outbreaks. The disease is presumably spread mainly by fecal-oral transmission, which explains in part its high frequency in childcare centers. Rotaviruses infect villous epithelial cells in the jejunum and ileum. Children with rotavirus infection typically present with fever, vomiting, and diarrhea. Dehydration can be significant. Adults remain susceptible to rotavirus infection, but serious morbidity and mortality is rare except among the elderly. Outbreaks of rotavirus diarrhea can occur in nursing homes, sometimes with dehydration and death.
|
||||
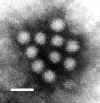 Figure .
Figure . Norwalk virus from stool sample from an individual with gastroenteritis. F.P. Williams, U.S. Environmental Protection Agency |
NOROVIRUSES AND OTHER CALICIVIRUSES Caliciviruses, so-named because of cup-life indentations on their surfaces (calix is Latin for “cup” or “goblet”) cause vomiting, diarrhea, or both. Noroviruses (previously called the Norwalk agent, after an outbreak in Norwalk, Ohio, led to the first isolation) and similar agents cause more than one-third of outbreaks of nonbacterial gastroenteritis in the U.S., affecting persons of all age groups. Some studies suggest that up to 90% of persons are eventually infected. Like rotaviruses, caliciviruses are spread mainly by fecal-oral transmission and infect villous cells in the small intestine, causing diarrhea as a result of malabsorption. Onset of illness can be gradual or abrupt, with crampy abdominal pain being the usual first symptom. Most patients develop both vomiting and diarrhea, but either of these symptoms can predominate. Some patients experience only mild watery diarrhea, while others have a more severe illness with vomiting, headache, and constitutional symptoms. Caliciviruses have been difficult to isolate by culture. No convenient diagnostic test is available, although numerous tests are in various stages of development. Treatment is supportive, and rapid recovery within 48 to 72 hours is the rule.
|
||||
|
Astroviruses and other agents of viral gastroenteritis Astroviruses and enteric (group F) adenoviruses have been firmly established as causes of diarrhea. Astroviruses cause diarrhea primarily in young children. Serologic studies indicate that the majority of children are infected by 6 years of age. Illness is usually manifested by headache, malaise, and diarrhea, with nausea being less common. The illness tends to be milder than rotavirus diarrhea, although occasional patients require hospitalization. Secondary cases in adult contacts are less common, and experimental studies indicate that astroviruses usually do not produce symptoms in adults. Both astroviruses and enteric adenoviruses have been isolated by culture. A commercially available test is available for detection of enteric adenoviruses, which also cause diarrheal disease in children. Other viruses that may sometimes cause diarrhea but in which the etiologic or causal relationship has not yet been firmly established include coronaviruses, echoviruses, coxsackie A and B viruses, non-group F adenoviruses, picobirnaviruses, picotrirnaviruses, pestiviruses, and toroviruses.
|
|||||
|
E. coli other than Shiga toxin-producing strains Escherichia coli, a major component of the normal intestinal flora and the most carefully studied of all living organisms, causes diarrhea by at least 6 mechanisms, identified by the adjectives used to describe the respective strains:
Diagnosis of E. coli as the cause of diarrhea in one of the latter syndromes is usually not accomplished by most clinical laboratories. Therefore, consultation with a state health department may be advisable when an outbreak of severe diarrhea occurs and cultures for the usual pathogens are unrevealing.
|
|||||
 Figure .
Figure . Vibrio cholerae - Gram-negative, facultatively anaerobic, curved (vibrio-shaped), rod prokaryote; causes Asiatic cholera. © Dennis Kunkel Microscopy, Inc. Used with permission |
Cholera and non-Cholera vibriosVibrio cholerae causes a life-threatening secretory diarrhea by stimulating the adenylate cyclase system of the gastrointestinal tract. The disease presents abruptly with watery diarrhea. Fever and abdominal pain are seldom prominent, if they occur at all. Death occurs by dehydration. Treatment requires aggressive fluid replacement, with antibiotics having a secondary role. Cholera is rare in the U.S., being seen almost exclusively in travelers, but the possibility of an eighth pandemic of the disease remains real. Vibrio parahemolyticus is an important cause of gastroenteritis associated with the ingestion of inadequately cooked seafood or food that has been contaminated with seawater. Raw oysters are incriminated in about one-half of cases in the Gulf Coast states, where the disease is most common in the U.S., but outbreaks are associated with other types of shellfish. Between 1973 and 1998, 40 outbreaks of V. parahemolyticus infection were to the CDC, accounted for > 1000 illness. Explosive watery diarrhea is usually the first symptom and is often accompanied by crampy abdominal pain. The organism can be isolated on TCBS agar. The disease is usually mild and self-limited, but occasional deaths occur in young children, elderly persons, or persons who are severely debilitated.
|
||||
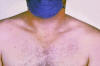 Figure
FigureRose spots on the chest of a patient with typhoid fever due to the bacterium Salmonella typhi. Symptoms of typhoid fever may include a sustained fever as high as 103° to 104° F (39° to 40° C), weakness, stomach pains, headache, loss of appetite. In some cases, patients have a rash of flat, rose-colored spots. CDC/Armed Forces Institute of Pathology, Charles N. Farmer |
Enteric Fever (Typhoid and Paratyphoid Fevers) The elimination of typhoid as a major public health problem in the U.S. was due largely to improved sanitation rather than to antibiotics or to the vaccine, which is only partially effective. Occasional outbreaks of typhoid fever still occur, but about three-fourths of cases are acquired abroad, and especially from Mexico, the Philippines, and India. The risk of typhoid among U.S. travelers is greatest among those who visit the Indian subcontinent. Paratyphoid fever is an enteric fever syndrome due to Salmonella species other than S. typhi. Typhoid fever is caused by Salmonella choleraesuis subspecies choleraesuis serotype typhi, commonly known as S. typhi. A similar illness is caused by Salmonella paratyphi A, Salmonella schottmuelleri (formerly S. paratyphi B), and S. hirschfeldii (formerly S. paratyphi C), and occasionally by other Salmonella species. Enteric fever is a “penetrating” intestinal infection in the sense that the bacteria penetrate the small bowel mucosa, where the multiply in intestinal lymphoid tissue and especially in the large aggregates of lymphocytes in the ileum known as Peyer’s patches. Lymphohematogenous dissemination results in high fever and other disease manifestations. The organisms grow within reticuloendothelial cells, and satisfactory host defenses require intact cell-mediated (T-lymphocyte) immunity. Typhoid fever usually affects persons < 30 years of age (children, adolescents, and young adults). After an incubation period of 5 to 21 days, patients present with abdominal pain, fever, chills, and constitutional symptoms. Diarrhea is seldom the presenting complaint, and about 30% of patients, especially adults, experience constipation rather than diarrhea. The illness characteristically evolves over several weeks:
Diagnosis is most conveniently made by blood cultures, which are positive in 40% to 80% of patients.
|
||||
Biliary Tract InfectionsCholecystitis and cholangitis are relatively common causes of acute, recurrent, and chronic abdominal pain. Occasional complications of biliary tract disease include acute pancreatitis caused by gallstones and liver abscess. Cholecystitis usually results from obstruction of the cystic duct with subsequent bacterial invasion of the gallbladder. In the U.S., cholelithiasis is the cause of cystic duct obstruction in > 90% of cases, and women are affected twice as frequently as men. Cholecystitis can be acute or chronic. More often than not, both types of inflammation are present, as evidence of chronic inflammation including fibrosis is found in about 95% of gallbladders removed for presumed acute cholecystitis. Acute cholecystitis, with or without gallstones, typically presents with pain in the right upper quadrant of the abdomen, fever, nausea, and vomiting. Common pathogens in biliary infections include aerobic gram-negative bacilli (e.g., E. coli, Klebsiella species, Enterobacter species, Proteus species, and Pseudomonas aeruginosa), aerobic gram-positive cocci (enterococci, streptococci, and staphylococci), and anaerobic bacteria (e.g., Bacteroides species, Clostridium species, Fusobacterium species, and peptostreptococci). Acute ascending cholangitis (also called toxic or suppurative cholangitis) occurs in the setting of common bile duct obstruction, most commonly from gallstones (choledocholithiasis). Other causes of common bile duct obstruction include benign or malignant strictures, extrinsic compression, and parasitic infestation (usually Ascaris lumbricodes in the U.S.). Patients with acute ascending cholangitis often have evidence of severe sepsis, and about 50% to 60% of patients manifest “Charcot’s triad” of fever, jaundice, and right upper quadrant abdominal pain.
|
|||||
|
Acute Appendicitis and Mesenteric Lymphadenitis (Pseudoappendicitis Syndrome) Acute appendicitis should be considered in all patients with fever, right lower quadrant abdominal pain, anorexia, and vomiting. However, these finding are nonspecific and the clinical presentation of acute appendicitis can be atypical. Several diseases closely mimic acute appendicitis including mesenteric lymphadenitis. Studies suggest that 8% of all persons and up to 20% of admitted to the hospital for suspected acute appendicitis may have mesenteric lymphadenitis instead.Acute appendicitis is usually caused by obstruction of the organ’s narrow lumen by fecaliths, foreign bodies, enlarged lymphatic follicles, or tumors. The mucosa and wall become ischemic, promoting bacterial invasion. Unchecked, the inflammatory process progresses to gangrene (infarction) and then perforation, allowing aerobic and anaerobic bacteria to enter the peritoneal cavity. Peritonitis can be localized as a right lower quadrant mass or abscess, or can be generalized. In either case, patients usually become septic.Mesenteric lymphadenitis (pseudoappendicitis) is most commonly caused by Yersinia enterocolitica and Yersinia pseudotuberculosis. Non-typhoid Salmonella species and Campylobacter jejuni have also been associated with this syndrome. Streptococci were not infrequently associated with this syndrome in the preantibiotic era. Unusual etiologies reported in recent years include infectious mononucleosis (Epstein-Barr virus) and intestinal anthrax.
|
|||||
|
Acute Diverticulitis Acute diverticulitis is a common problem in primary care because of the high prevalence in our society of diverticulosis of the colon. Acute diverticulosis is often self-limited, but can lead to serious complications including sepsis, intraabdominal abscess formation, and death. Acute diverticulitis is actually a “peridiverticulitis” in that the inflammatory reaction is almost entirely extrinsic to the colon. Microperforations of diverticula cause contamination of the peritoneal cavity by the aerobic and anaerobic flora resident to the normal colon (notably, Bacteroides species, E. coli, and enterococci). The result is often a small abscess within the pericolonic fat (Stage 1) that, if not contained, may expand and spread (Stage 2) and then rupture, causing generalized suppurative peritonitis (Stage 3). If the bowel lumen communicates with the inflammatory process, more colonic contents enter the peritoneal cavity leading to extensive fecal contamination of the peritoneal cavity and intraabdominal abscesses (Stage 4).
|
|||||
PeritonitisIntraabdominal infections can be confined to the peritoneal cavity, to one or another organ, or, more commonly involve both. Infectious peritonitis can be diffuse or localized. Examples of localized peritonitis secondary to acute cholecystitis, acute appendicitis, and acute diverticulitis have already been discussed. Diffuse (generalized) peritonitis and intraabdominal abscess nearly always mandate admission to the hospital. Primary peritonitis (spontaneous bacterial peritonitis) occurs in up to 10% of patients with alcoholic cirrhosis) and occurs rarely in patients with ascites caused by congestive heart failure, nephritic syndrome, metastatic tumors, systemic lupus erythematosus, or other disorders. E. coli is the most common infecting bacterium. Anaerobic bacteria are rare in primary peritonitis. Peritonitis can also be caused by tuberculosis, Neisseria gonorrhoeae and Chlamydia trachomatis infections. Secondary peritonitis usually results form spillage of bacteria from the gastrointestinal tract, as occurs in acute appendicitis with rupture, acute diverticulitis, ischemic bowel disease, intestinal obstruction due to neoplasm, and penetrating injuries. Other causes of secondary peritonitis include acute suppurative cholecystitis (see above), chronic ambulatory peritoneal dialysis, complicated genitourinary tract infections, and ruptured abscess from an intraabdominal organ such as the liver, pancreas, kidney, or fallopian tube. Secondary peritonitis typically involves both aerobic and anaerobic bacteria. Intraabdominal abscesses arise from one of several mechanisms: (1) local spread of infection within the peritoneal cavity; (2) hematogenous seeding of an organ; and (3) necrosis of an organ followed by bacterial colonization and superinfection.
|
|||||
|
|||||