- isoniazid
- rifampin
- ethambutol
- pyrazinamide
| x | x | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| INFECTIOUS DISEASE | BACTERIOLOGY | IMMUNOLOGY | MYCOLOGY | PARASITOLOGY | VIROLOGY | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SPANISH | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ALBANIAN | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Let us know what you think FEEDBACK |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SEARCH | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Logo image © Jeffrey Nelson, Rush University, Chicago, Illinois and The MicrobeLibrary | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
MYCOBACTERIA In the 1980's, many experts felt that the days of tuberculosis as a threat to the US population had passed and the incidence of new cases (around 20,000 a year) was slowly decreasing, even though it was still the leading infectious cause of death world-wide. The situation in the 1990's has changed dramatically. The incidence of tuberculosis has slightly increased and the disease is certainly not going away (This is primarily due to the AIDS epidemic). At the same time multiple drug-resistant strains of M. tuberculosis are appearing regularly. The M. avium - M. intracellulare complex, long considered a group of organisms that only rarely infects man, is now recognized as one of the leading opportunists associated with AIDS. M. leprae is the causative agent of leprosy which remains a major disease in the third world. Due to eradication of infected cattle and pasteurization of milk M. bovis (a zoonotic cause of tuberculosis) is rarely seen in the United States. Mycobacteria are obligate aerobic, acid-fast rods.
Mycobacterium tuberculosis Tuberculosis is extremely common in third world countries and WHO estimates that about one third of the world’s population is infected, although the rate of mortality due to tuberculosis around the world has fallen by 45% between 1990 and 2012. It is estimated that around the world in 2012:
More data from WHO are given in figure 5. In the United States there were 9,945 cases of tuberculosis in 2012
(3.2 cases peer 100,000 population). There was a decline of 5.4% in the number
of cases over those reported in 2011 (figure 1). In 1953, there were 84,304
cases of tuberculosis (a case rate of 52.6 per 100,000 population). Rates were
higher in the older population (figure 2) and an increasing proportion of cases
is seen in foreign-born persons (figures 3 and 4). In the United States in 2013,
64% of tuberculosis cases and 91% of multidrug–resistant cases occurred in
people born in other countries. Three quarters of these cases came from 15
countries.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
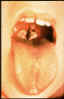
 Figure 6
Figure 6Mantoux intradermal tuberculin skin test for tuberculosis. CDC |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Atypicals The "atypicals" generally infect the immunocompromised host and are thus not transmitted man-man. With the AIDS epidemic, the atypical mycobacteria have taken on new importance with the recognition that the M. avium complex (MAC) results in the most commonly associated systemic bacterial infection. Atypical mycobacteria can cause tuberculosis-like or leprosy-like, diseases, and are not susceptible to certain common anti-tuberculous antibiotics.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
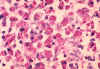
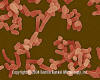 Mycobacterium avium. Rod-shaped Bacterium (causes avian tuberculosis)
(SEM x24,000) ©
Dennis Kunkel Microscopy, Inc.
Used with permission
Mycobacterium avium. Rod-shaped Bacterium (causes avian tuberculosis)
(SEM x24,000) ©
Dennis Kunkel Microscopy, Inc.
Used with permission
|
Mycobacterium avium complex and AIDS
M. avium generally infects AIDS patients when their CD4+ cell count decreases greatly (below 100/mm3). M. tuberculosis infects AIDS patients much earlier in the disease. This clearly demonstrates the much greater virulence of M. tuberculosis. The incidence of systemic disease (versus primarily pulmonary) is much greater in tuberculosis associated with AIDS than in its absence. Furthermore, histologically lesions often appear lepromatous (non-granulomatous, with many organisms). It is rare to find a case of M. avium infection that is not AIDS associated. However, M. tuberculosis is a much more virulent organism. Approximately 20% of the total tuberculosis cases in the US are caused by AIDS. This helps explain why TB is no longer on the decline. Increased homelessness is also suggested to be a factor in the rise of tuberculosis. Treatment of M. avium also involves a long-term regimen of multiple drug combinations. However, this organism does not always respond to the drug regimens used to treat M. tuberculosis. Appropriate drug combinations are still under investigation in clinical trials. Since M. tuberculosis is the more virulent organism, the drug regimen selected is primarily against M. tuberculosis. If M. avium is suspected other agents effective against this organism are included. Other atypicals Presence or absence of pigmentation (and its dependence on growth in the light) and slow or fast growth rates of atypical mycobacteria allow some differentiation - the "Runyon Groups". Modern techniques allow ready speciation of mycobacteria based on their cellular fatty acid and/or mycolic acid profiles. This is only performed in reference laboratories. Mycolic acids are components of a variety of lipids found only in mycobacteria, nocardia and corynebacteria. The chain length of these mycolic acids is longest in mycobacteria, intermediate in nocardia and shortest in corynebacteria. This explains why mycobacteria are generally acid fast; nocardia less acid fast; and corynebacteria are non-acid fast.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
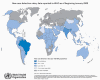
 Global Leprosy Situation 2009 © World Health
Organization
Global Leprosy Situation 2009 © World Health
Organization
|
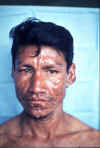
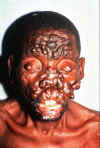
Mycobacterium leprae M. leprae is the causative agent of leprosy (Hansen's Disease), a chronic disease often leading to disfigurement.. It is rarely seen in the U.S. but common in the third world. The organism infects the skin, because of its growth at low temperature. It also has a strong affinity for nerves. In "tuberculoid" leprosy, there are few organisms due to control by active cell-mediated immunity. In "lepromatous" leprosy, due to immunosuppression by the organism, the opposite is found. Although uncommon in the U.S., millions of cases occur worldwide. Treatment with antibiotics (initially dapsone and now multi-drug) is effective and the overall disease incidence worldwide is down. The organism does not grow in culture media. However, it grows well in the armadillo (which has a low body temperature), allowing production of M. leprae antigens and pathogenesis studies. M. leprae has traditionally been identified on the basis of acid-fast stains of skin biopsies and clinical picture. Lepromin is used in skin testing.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Corynebacterium diphtheriae. Rod,clubed-shaped Bacterium (causes diphtheria),
(SEM x24,000) ©
Dennis Kunkel Microscopy, Inc.
Used with permission
Corynebacterium diphtheriae. Rod,clubed-shaped Bacterium (causes diphtheria),
(SEM x24,000) ©
Dennis Kunkel Microscopy, Inc.
Used with permission
|
CORYNEBACTERIA Corynebacterium diphtheriae C. diphtheriae grows best under strict aerobic conditions It is Gram positive and pleomorphic. Colonization of the upper respiratory tract (pharynx and nose) and less commonly skin with C. diphtheriae can lead to diphtheria. The organism does not produce a systemic infection. However, in addition to a pseudomembrane being formed locally (which can cause choking), systemic and fatal injury results primarily from circulation of the potent exotoxin (diphtheria toxin). The latter begins over a period of a week. Thus treatment involves rapid therapy with anti-toxin. The gene for toxin synthesis is encoded on a bacteriophage (the tox gene). Corynebacteria that are not infected with phage, thus do not generally cause diphtheria. Diphtheria is now a disease of almost historic importance in the U.S. due to effective immunization of infants (in conjunction with pertussis and tetanus, DPT) with a toxoid (inactive toxin) which causes production of neutralizing antibodies. However, colonization is not inhibited and thus C. diphtheriae is still found in the normal flora (i.e. a carrier state exists). Immunity can be monitored with the Schick skin test. Treatment in non-immune individuals primarily involves injection of anti-toxin. Antibiotics are also administered at this time. The toxin consists of two types of polypeptide. One binds to host cells; the other then becomes internalized and inhibits protein synthesis. The exotoxin catalyses the covalent attachment of the ADP-ribose moiety of NADH to a rare amino acid, diphthamide, present in EF2 (elongation factor 2). The toxin is not synthesized in the presence of iron as an iron-repressor complex forms which inhibits expression of the tox gene. C. diphtheriae are identified by growth on Loeffler's medium followed by staining for metachromatic bodies (polyphosphate granules, Babes-Ernst bodies). The term "metachromatic" refers to the color difference of the intracellular polyphosphate granules (pink) compared to the rest of the cell (blue). Characteristic black colonies are seen on tellurite agar from precipitation of tellurium on reduction by the bacteria. Production of exotoxin can be determined by in vivo or in vitro tests. Other organisms which morphologically resemble C. diphtheriae ("diphtheroids" which include other corynebacteria and also propionibacteria) are found in the normal flora. Isolates should not be confused with these organisms.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||