|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
PORTUGUESE |
Please Note: A new expanded and
updated version of this page is now available
Please go
here
MYCOLOGY - CHAPTER ONE
INTRODUCTION TO MYCOLOGY
Dr Art DiSalvo
Emeritus Director, Nevada State Laboratory
Emeritus Director of Laboratories, South Carolina Department of Health and
Environmental Control
|
|
TURKISH |
|
ALBANIAN |
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|
|
|
 |
|
|
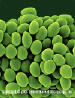 Figure 1. Chaetomium globosum spores. Chaetomium is an ascomycete, and
in most species the spores are lemon-shaped, with a single germ pore
Figure 1. Chaetomium globosum spores. Chaetomium is an ascomycete, and
in most species the spores are lemon-shaped, with a single germ pore
©
Dennis Kunkel Microscopy, Inc. Used with permission
 A
A
 B
B
Figure 2.
A. Bracket fungus basidiocarp (fruiting body). B. Lower surface showing
generative hyphae. Reproductive spores are
dispersed through pores in the surface of the brackets.
© Dr Arthur DiSalvo and ©
Dennis Kunkel Microscopy, Inc. Used with permission
|
INTRODUCTION
Classification
Fungi are eukaryotic
organisms that do not contain chlorophyll, but have cell walls, filamentous
structures, and produce spores. These organisms grow as saprophytes and
decompose dead organic matter. There are between 100,000 to 200,000 species
depending on how they are classified. About 300 species are presently known
to be pathogenic for man.
There are five kingdoms
of living things. The fungi are in the Kingdom Fungi.
|
KINGDOM |
CHARACTERISTIC |
EXAMPLE |
|
Monera |
Prokaryocyte |
Bacteria
Actinomycetes
|
|
Protista |
Eukaryocyte |
Protozoa |
|
Fungi |
Eukaryocyte * |
Fungi |
|
Plantae |
Eukaryocyte |
Plants, Moss |
|
Animalia |
Eukaryocyte * |
Arthropods
Mammals
Man
|
*This common
characteristic is responsible for the therapeutic dilemma in anti-mycotic
therapy.
The taxonomy of the
Kingdom Fungi is evolving and is controversial. Formerly based on gross and
light microscopic morphology, studies of ultra structure, biochemistry and
molecular biology provide new evidence on which to base taxonomic positions.
Medically important fungi are in four phyla:
-
Ascomycota - Sexual
reproduction in a sack called an ascus with the production of ascopspores
(figure 1).
-
Basidiomycota - Sexual
reproduction in a sack called a basidium with the production of basidiospores
(figure 2).
-
Zygomycota - sexual
reproduction by gametes and asexual reproduction with the formation of
zygospores (figure 3).
-
Mitosporic Fungi (Fungi
Imperfecti) - no recognizable form of sexual reproduction. Includes most
pathogenic fungi.
|
| |
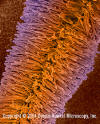
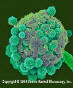 Figure 3.
Figure 3.
Mucor spp. fruiting structure with spores. The fruiting structure (condiophore)
has matured and its outer membrane is disintegrating allowing the spores
(conidia) to be released. Mucor is a common fungus found in many
environments. It is a Zygomycetes fungus which may be allergenic and is
often found as saprobes in soils, dead plant material (such as hay), horse
dung, and fruits. It is an opportunistic pathogen and may cause mucorosis
in immuno-compromised individuals. The sites of infections are the lung,
nasal sinus, brain, eye, and skin. Few species have been isolated from
cases of zygomycosis, but the term mucormycosis has often been used.
Zygomycosis includes mucocutaneous and rhinocerebral infections, as well
as renal infections, gastritis, and pulmonary infections.
©
Dennis Kunkel Microscopy, Inc. Used with permission
|
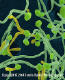 Figure 4.
Figure 4.
Candida albicans - yeast and hyphae stages. A yeast-like fungus
commonly occuring on human skin, in the upper respiratory, alimentary &
female genital tracts. This fungus has a dimorphic life cycle with yeast
and hyphal stages. The yeast produces hyphae (strands) and pseudohyphae.
The pseudohyphae can give rise to yeast cells by apical or lateral
budding. Causes candidiasis which includes thrush (an infection of the
mouth & vagina) and vulvo-vaginitis.
©
Dennis Kunkel Microscopy, Inc. Used with permission |
MORPHOLOGY
Pathogenic fungi can exist as
yeasts or as hyphae (figure 4). A mass of hyphae is called mycelia. Yeasts are unicellular
organisms and mycelia are multicellular filamentous structures, constituted by
tubular cells with cell walls. The yeasts reproduce by budding. The mycelial
forms branch and the pattern of branching is an aid to morphological
identification. If the mycelia do not have
septa, they are called coenocytic (non-septate).
The terms "hypha" and "mycelium" are frequently used
interchangeably. Some fungi occur in both the yeast and mycelial forms. These
are called dimorphic fungi.
Dimorphic fungi
The dimorphic fungi have two
forms (figure 5):
-
YEAST - (parasitic
or pathogenic form). This is the form usually seen in tissue, in exudates, or if
cultured in an incubator at 37 degrees C.
-
MYCELIUM -
(saprophytic form). The form observed in nature or when cultured at 25 degrees
C.
Conversion to the yeast form appears to be essential for pathogenicity. Dimorphic fungi are identified by several morphological or biochemical
characteristics, including the appearance of their fruiting bodies. The asexual
spores may be large (macroconidia, chlamydospores) or small (microconidia,
blastospores, arthroconidia).
MYCOTIC DISEASES
There are four types of
mycotic diseases:
-
Hypersensitivity - an
allergic reaction to molds and spores
-
Mycotoxicoses - poisoning
of man and animals by food products contaminated by fungi which
produce toxins from the grain substrate
-
Mycetismus - the ingestion
of toxin (mushroom poisoning)
-
Infection - tissue
invasion with a host response
We shall be concerned only
with the last type: pathogenic fungi that cause infections. Most common
pathogenic fungi do not produce toxins but they do cause physiologic modifications
during a parasitic infection (e.g., increased metabolic rate, modified metabolic
pathways and modified cell wall structure). The mechanisms that cause these
modifications, as well as their significance as a pathogenic mechanism, are just
being described.
Most pathogenic fungi are also thermotolerant, and can resist
the effects of the active oxygen radicals released during the respiratory burst
of phagocytes. Thus, fungi are able to withstand many host defenses. Fungi are
ubiquitous in nature and most people are exposed to them. The establishment of a
mycotic infection usually depends on the size of the inoculum and on the
resistance of the host. The severity of the infection seems to depend mostly on
the immunologic status of the host. Thus, the demonstration of fungi, for
example, in blood drawn from an intravenous catheter can correspond to
colonization of the catheter, to transient fungemia (i.e., dissemination of
fungi through the blood stream), or to a true infection. The physician must
decide which is the clinical status of the patient based on clinical parameters,
general status of the patient, laboratory results, etc. The decision is not
trivial, since treatment of systemic fungal infections requires the aggressive
use of drugs with considerable toxicity. Most mycotic agents are soil
saprophytes and mycotic diseases are generally not communicable from
person-to-person (occasional exceptions are: Candida and some dermatophytes).
Outbreaks of disease may occur, but these are due to a common environmental
exposure, not communicability. Most of the fungi which cause systemic infections
have a peculiar, characteristic ecologic niche in nature. This habitat is
specific for several fungi which will be discussed later. In this environment,
the normally saprophytic organisms proliferate and develop. This habitat is also
the source of fungal elements and/or spores, where man and animals, incidental
hosts, are exposed to the infectious particles. It is important to be aware of
these associations to diagnose mycotic diseases. The physician must be able to
elicit a complete history from the patient including occupation, avocation and
travel history. This information is frequently required to raise, or confirm,
a differential diagnosis. The incidence of mycotic infections is currently
increasing dramatically, due to an increased population of susceptibles.
Examples are patients with AIDS, patients on immunosuppressive therapy, and the use of
more invasive diagnostic and surgical procedures (prosthetic implants). Fungal
diseases are non-contagious and non-reportable diseases in the national public
health statistics.
|
|
|
|
VIDEO
Growth and Division of Budding Yeast (Saccharomyces
cerevisiae)
High Resolution
Low resolution
© Philip Meaden
Heriot-Watt University
Edinburgh, Scotland and The
MicrobeLibrary
|
 A
A
Candida albicans is a
dimorphic fungus in that it grows as a unicellular yeast under some
environmental conditions and as a filamentous fungus under other
conditions.
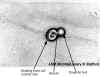
Budding yeast cells. C. albicans was grown at 37°C with aeration
for 3 h in yeast-peptone-dextrose (YPD) medium. In this image, unstained
cells are magnified x400. The image was taken with phase- contrast
microscopy. |
 B
B
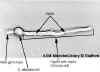
Budding yeast with septum. The septum has formed between the daughter bud and the mother cell, but separation of the two has not occurred. This image is from a culture of cells grown at 37° C for 3 h in YPD medium. The unstained cell is magnified x1,000 using
phase- contrast microscopy.
 C
C
C. albicans cell at 3 h. Three hours after the appearance of the germ tube, the hypha has septa. A new germ tube at the distal pole of the
cell is also evident at this time. The unstained cells are magnified x1,000 using phase-contrast microscopy.
|
Figure 5 A-C
© Phillip Stafford
Dartmouth Medical School
Hanover, New Hampshire and
The
MicrobeLibrary |
 Figure 6
Figure 6
Gomori staining technique, and under a relatively low magnification of
50X, this photomicrograph reveals histopathologic changes indicative of
the presence of the dematiaceous fungal organism, Phialophora parasitica.
Known to be a causative agent for chromoblastomycosis and
phaeohyphomycosis, which affect the subcutaneous tissues, however, in
the case of phaeohyphomycosis, many organ systems may be affected, even
becoming disseminated throughout the body.
CDC/ Dr. L. Ajello
 Figure 7
Figure 7
A Sabouraud’s dextrose agar plate culture growing a Mexican isolate of
T. rubrum var. rodhaini. Dermatophytic members of the genus
Trichophyton are some of the leading causes of hair, skin, and nail
infections in humans, known as dermatophytoses. The genus includes
anthropophilic, zoophilic, and geophilic species
CDC/Dr. Libero Ajello |
DIAGNOSIS
-
Skin scrapings suspected
to contain dermatophytes or pus from a lesion can be mounted in KOH on a
slide (wet preparation) and examined directly under the microscope.
-
Skin testing (dermal
hypersensitivity) used to be popular as a diagnostic tool, but this is now
discouraged because the skin test may interfere with serological studies, by
causing false positive results. It may still be used to evaluate the
patient's immunity, as well as a population exposure index in
epidemiological studies.
-
Serology may be helpful
when it is applied to a specific fungal disease; there are no screening
antigens for 'fungi' in general. Because fungi are poor antigens, the
efficacy of serology varies with different fungal infections. The serologic
tests will be discussed under each mycosis. The most common serological
tests for fungi are based on double
immunodiffusion,
complement fixation and enzyme immunoassays
(EIA). Double immunodiffusion and complement fixation usually detect IgG
antibodies. Some EIA tests are being developed to detect both IgG and IgM
antibodies. There are some tests that can detect specific fungal antigens,
but they are just coming into general use.
-
Direct fluorescence
microscopy may be used for identification, even on non-viable cultures or on
fixed tissue sections. The reagents for this test are difficult to obtain.
-
Biopsy and
histopathology. A biopsy may be very useful for the identification and
as a source of the of tissue-invading fungi. Usually the Gomori methenamine
silver (GMS) stain is used to reveal the organisms which stain black against
a green background (figure 6). The H&E stain does not always tint the
organism, but it will stain the inflammatory cells.
-
Culture. A
definitive diagnosis requires a culture and identification. Pathogenic fungi
are usually grown on Sabouraud dextrose agar (figure 7). It has a slightly
acidic pH (~5.6). Cycloheximide, penicillin, streptomycin or other
inhibitory substances are often added to prevent bacterial contamination and
overgrowth. Two cultures are inoculated and incubated separately at 25
degrees C and 37 degrees C to reveal dimorphism. The cultures are examined
macroscopically and microscopically. They are not considered negative for
growth until after 4 weeks of incubation.
-
DNA probes. Ribosomal DNA is
hybridized to a labeled DNA probe. This test is rapid (1 to 2 hours) and
species-specific. It is not available for many organisms and it is
expensive.
|
|
MOLECULAR STRUCTURE
Amphotericin B
Ketoconazole
Griseofulvin
5-fluorocytosine
|
TREATMENT
Mammalian cells
do not contain the enzymes that will degrade the cell wall polysaccharides of
fungi. Therefore, these pathogens are difficult to eradicate by the animal host
defense mechanisms. Because mammals and fungi are both eukaryotic, the cellular
milieu is biochemically similar in both. The cell membranes of all eukaryotic
cells contain sterols; ergosterol in the fungal cell membrane and cholesterol in
the mammalian cell membrane. Thus, most substances which may impair the invading
fungus will usually have serious side effects on the host. Although one of the
first chemotherapeutic agents (oral iodides) was an anti-mycotic used in 1903,
the further development of such agents has been left far behind the development
of anti-bacterial agents. The selective toxicity necessary to inhibit the
invading organism with minimal damage to the host has been difficult to
establish within eukaryotic cells.
The primary antifungal agents
are:
Amphotericin B
This is a polyene antimycotic. It is usually the drug of choice for most systemic fungal infections.
It has a greater affinity for ergosterol in the cell membranes of fungi than for
the cholesterol in the host's cells; once bound to ergosterol, it causes
disruption of the cell membrane and death of the fungal cell. Amphotericin B is
usually administered intravenously and patients are usually hospitalized. The drug is rather toxic; thrombo-phlebitis,
nephrotoxicity, fever, chills and anemia frequently occur during administration.
Lipid-based Amphotericin B is as effective less toxic and more expensive.
Azoles
The azoles (imidazoles and
triazoles), including ketoconazole, fluconazole, itraconozole, voriconazole and
posaconazole are being
used for muco-cutaneous candidiasis, dermatophytosis, and for some systemic
fungal infections. Fluconazole is presently essential for the maintenance of
AIDS patients with cryptococcosis. The general mechanism of action of the azoles
is the inhibition of ergosterol synthesis. Oral administration and reduced
toxicity are distinct advantages.
Griseofulvin
Griseofulvin is a very
slow-acting drug which is used for severe skin and nail infections. Its effect
depends on its accumulation in the stratum corneum where it is incorporated into
the tissue and forms a barrier which stops further fungal penetration and
growth. It is administered orally. The exact mechanism of action is unknown.
5-fluorocytosine
5-fluorocytosine (Flucytosine
or 5-FC) inhibits RNA synthesis and has found its main application in
cryptococcosis (to be discussed later). It is administered orally.
Terbinofine
(E)-N-(6,6-dimethyl-2-hepten-4-ynyl)-N-methyl-1-naphthalenemethanamine
hydrochloride ( terbinafine hydrochloride).
This is an anti-fungal agent, also
known as Lamisil, used to treat infections of fingernails and toenails. It
is taken orally.
Caspofungin
1-[(4R,5S)-5-[(2-aminoethyl)amino]-
N2-(10,12-dimethyl-1-oxotetradecyl)- 4-hydroxy-L-ornithine]-5-[(3R)-
3-hydroxy-L-ornithine] pneumocandin B0.
This anti-fungal works by inhibiting
the enzyme β(1,3)-D-Glucan synthase and altering the integrity of the fungal
cell wall. It is administered intravenously.
CLINICAL CLASSIFICATION OF
THE MYCOSES
Fungal diseases may be discussed in a variety of ways. The most
practical method for medical students is the clinical taxonomy which divides the
fungi into:
-
Superficial mycoses
-
Subcutaneous
mycoses
-
Systemic mycoses
-
Opportunistic
mycoses
The superficial mycoses (or cutaneous mycoses) are fungal diseases that are confined to the outer layers of
the skin, nail, or hair, (keratinized layers) rarely invading the deeper tissue
or viscera (figure 8). The fungi involved are called dermatophytes. The subcutaneous
mycoses are confined to the subcutaneous tissue and only rarely spread
systemically. They usually form deep, ulcerated skin lesions or fungating
masses, most commonly involving the lower extremities. The causative organisms
are soil saprophytes which are introduced through trauma to the feet or legs.
The
systemic mycoses may involve deep viscera and become widely disseminated. Each
fungus type has its own predilection for various organs which will be described
as we discuss the individual diseases.
The opportunistic mycoses are
infections due to fungi with low inherent virulence. The etiologic agents are
organisms which are common in all environments.
|
|
|
|
MOLECULAR
STRUCTURE
Ergosterol
Caspofungin |
|
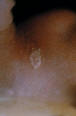 Figure 8.
Figure 8.
Ringworm on the skin of the neck due to Trichophyton rubrum.
CDC/Lucille K. Georg
|
|
|
 Return to the Mycology Section of Microbiology and Immunology On-line
Return to the Mycology Section of Microbiology and Immunology On-line
This page last changed on
Sunday, December 30, 2018
Page maintained by
Richard Hunt
|

 Figure 1. Chaetomium globosum spores. Chaetomium is an ascomycete, and
in most species the spores are lemon-shaped, with a single germ pore
Figure 1. Chaetomium globosum spores. Chaetomium is an ascomycete, and
in most species the spores are lemon-shaped, with a single germ pore Figure 4.
Figure 4.  A
A  Figure 6
Figure 6






