|
Dr Alvin Fox Medical Microbiology, MBIM 650/720 READING: Murray, Third Edition Chapter 40 (mycobacteria) Chapter 26 (Corynebacterium diphtheriae) and Chapter 35 (Legionella) |
BACTERIOLOGY - LECTURE SIXTEEN MYCOBACTERIA, CORYNEBACTERIA AND LEGIONELLA
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
KEYWORDS |
MYCOBACTERIA In the 1980's, many experts felt that the days of tuberculosis as a threat to the US population had passed and the incidence of new cases (around 20,000 a year) was slowly decreasing, even though it was still the leading infectious cause of death world-wide. The situation in the 1990's has changed dramatically. The incidence of tuberculosis has slightly increased and the disease is certainly not going away (This is primarily due to the AIDS epidemic). At the same time multiple drug-resistant strains of M. tuberculosis are appearing regularly. The M. avium - M. intracellulare complex, long considered a group of organisms that only rarely infects man, is now recognized as one of the leading opportunists associated with AIDS. M. leprae is the causative agent of leprosy which remains a major disease in the third world. Due to eradication of infected cattle and pasteurization of milk M. bovis (a zoonotic cause of tuberculosis) is rarely seen in the United States. Mycobacteria are obligate aerobic, acid-fast rods.
Mycobacterium tuberculosis Pathogenesis of tuberculosis Mycobacterium tuberculosis infects the lung, and is distributed systemically within macrophages and survives intracellularly. Inhibition of phagosome-lysosome fusion and resistance to lysosomal enzymes have both been suggested to play a role. Cell-mediated immunity develops which causes infiltration of macrophages and lymphocytes with development of granulomas (tubercles). The disease can be diagnosed by skin testing for delayed hypersensitivity with tuberculin (also know as protein purified purified from Mycobacterium tuberculosis, PPD). A positive test does not indicate active disease; merely exposure to the organism. Other pathogenesis factors (of considerably less importance than delayed hypersensitivity) include mycobactin (a siderophore) and cord factor which damages mitochondria. Diagnosis, identification and treatment The presence of acid fast bacteria in sputum is a rapid presumptive test for tuberculosis (link to method). Subsequently, when cultured, M. tuberculosis will grow very slowly producing distinct non-pigmented colonies after several weeks. M. tuberculosis can be differentiated from most other mycobacteria by the production of niacin. A rapid alternative to culture is polymerase chain amplification (PCR). Tuberculosis is usually treated for extensive time periods (9 months or longer) since the organism grows slowly and may become dormant. By using two or more antibiotics (including rifampin and isoniazid), the possibility of resistance developing during this extended time is minimized. M. tuberculosis causes disease in healthy individuals and is transmitted man-man in airborne droplets. Vaccination The BCG vaccine (Bacillus de Calmette et Guerin, an attenuated strain of M. bovis) has not been effective. In the US, where the incidence of tuberculosis is low, widespread vaccination is not practiced. Indeed immunization (resulting in a positive PPD test) is felt to interfere with diagnosis. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
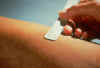 Mantoux intradermal tuberculin skin test for tuberculosis.
CDC
Mantoux intradermal tuberculin skin test for tuberculosis.
CDC
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
WEB RESOURCES Acid-Fast staining procedure for mycobacteria CDC
Tuberculisis FAQ |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
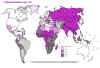 Estimated TB incidence rates 1997 WHO
Estimated TB incidence rates 1997 WHO |
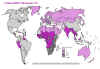 Estimated HIV/TB co-infection rates, 1997 WHO
Estimated HIV/TB co-infection rates, 1997 WHO |
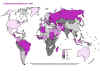 Tuberculosis notification rates, 1998 WHO
Tuberculosis notification rates, 1998 WHO |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Mycobacterium tuberculosis. Rod-shaped Bacterium (SEM x40,000) ©
Dr
Dennis
Kunkel, University of Hawaii. Used with permission
Mycobacterium tuberculosis. Rod-shaped Bacterium (SEM x40,000) ©
Dr
Dennis
Kunkel, University of Hawaii. Used with permission |
Atypicals The "atypicals" generally infect the immunocompromised host and are thus not transmitted man-man. With the AIDS epidemic, the atypical mycobacteria have taken on new importance with the recognition that the M. avium complex (MAC) results in the most commonly associated systemic bacterial infection. Atypical mycobacteria can cause tuberculosis-like or leprosy-like, diseases, and are not susceptible to certain common anti-tuberculous antibiotics.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Mycobacterium avium. Rod-shaped Bacterium (causes avian tuberculosis)
(SEM x24,000) Copyright Dr
Dennis
Kunkel, University of Hawaii. Used with permission
Mycobacterium avium. Rod-shaped Bacterium (causes avian tuberculosis)
(SEM x24,000) Copyright Dr
Dennis
Kunkel, University of Hawaii. Used with permission
|
Mycobacterium avium complex and AIDS
M. avium generally infects AIDS patients when their CD4+ cell count decreases greatly (below 100/mm3). M. tuberculosis infects AIDS patients much earlier in the disease. This clearly demonstrates the much greater virulence of M. tuberculosis. The incidence of systemic disease (versus primarily pulmonary) is much greater in tuberculosis associated with AIDS than in its absence. Furthermore, histologically lesions often appear lepromatous (non-granulomatous, with many organisms). It is rare to find a case of M. avium infection that is not AIDS associated. However, M. tuberculosis is a much more virulent organism. Approximately 20% of the total tuberculosis cases in the US are caused by AIDS. This helps explain why TB is no longer on the decline. Increased homelessness is also suggested to be a factor in the rise of tuberculosis. Treatment of M. avium also involves a long-term regimen of multiple drug combinations. However, this organism does not always respond to the drug regimens used to treat M. tuberculosis. Appropriate drug combinations are still under investigation in clinical trials. Since M. tuberculosis is the more virulent organism, the drug regimen selected is primarily against M. tuberculosis. If M. avium is suspected other agents effective against this organism are included. Other atypicals Presence or absence of pigmentation (and its dependence on growth in the light) and slow or fast growth rates of atypical mycobacteria allow some differentiation - the "Runyon Groups". Modern techniques allow ready speciation of mycobacteria based on their cellular fatty acid and/or mycolic acid profiles. This is only performed in reference laboratories. Mycolic acids are components of a variety of lipids found only in mycobacteria, nocardia and corynebacteria. The chain length of these mycolic acids is longest in mycobacteria, intermediate in nocardia and shortest in corynebacteria. This explains why mycobacteria are generally acid fast; nocardia less acid fast; and corynebacteria are non-acid fast.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Global Leprosy Situation 1998 © World Health
Organization
Global Leprosy Situation 1998 © World Health
Organization
|
Mycobacterium
leprae
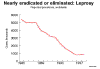
M. leprae is the causative agent of leprosy (Hansen's Disease), a chronic disease often leading to disfigurement.. It is rarely seen in the U.S. but common in the third world. The organism infects the skin, because of its growth at low temperature. It also has a strong affinity for nerves. In "tuberculoid" leprosy, there are few organisms due to control by active cell-mediated immunity. In "lepromatous" leprosy, due to immunosuppression by the organism, the opposite is found. Although uncommon in the U.S., millions of cases occur worldwide. Treatment with antibiotics (initially dapsone and now multi-drug) is effective and the overall disease incidence worldwide is down. The organism does not grow in culture media. However, it grows well in the armadillo (which has a low body temperature), allowing production of M. leprae antigens and pathogenesis studies. M. leprae has traditionally been identified on the basis of acid-fast stains of skin biopsies and clinical picture. Lepromin is used in skin testing. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
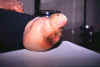 Azadegan Clinic, Teheran: The foot of a woman that has been grossly disfigured through leprosy
infection. © World Health Organization/TDR/Crump)
Azadegan Clinic, Teheran: The foot of a woman that has been grossly disfigured through leprosy
infection. © World Health Organization/TDR/Crump) |
 Deformity due to nerve damage with its consequent ulcers and resorption of bone. Such deformities can be worsened by careless use of the hands.
© WHO/TDR
Deformity due to nerve damage with its consequent ulcers and resorption of bone. Such deformities can be worsened by careless use of the hands.
© WHO/TDR |
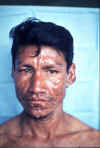 The face of a Peruvian man with active lepromatous
leprosy.
© WHO/ TDR/ McDougall
The face of a Peruvian man with active lepromatous
leprosy.
© WHO/ TDR/ McDougall |
 A young girl, 8 years old, with Burmese-Scots ancestry. The loss of eyebrows is an indication of diffuse lepromatous leprosy
©WHO/ TDR/ McDougall
A young girl, 8 years old, with Burmese-Scots ancestry. The loss of eyebrows is an indication of diffuse lepromatous leprosy
©WHO/ TDR/ McDougall |
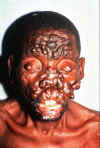 The face of a patient with active, neglected nodulous lepromatous leprosy. With treatment, all nodules could be
reversed.
©WHO/TDR/McDougall
The face of a patient with active, neglected nodulous lepromatous leprosy. With treatment, all nodules could be
reversed.
©WHO/TDR/McDougall |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 The trunk of a patient showing abnormal lesions of
dapsone-resistant leprosy ©WHO/TDR/McDougall
The trunk of a patient showing abnormal lesions of
dapsone-resistant leprosy ©WHO/TDR/McDougall |
WEB RESOURCES |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Corynebacterium diphtheriae. Rod,clubed-shaped Bacterium (causes diphtheria),
(SEM x24,000) Copyright Dr
Dennis
Kunkel, University of Hawaii. Used with permission
Corynebacterium diphtheriae. Rod,clubed-shaped Bacterium (causes diphtheria),
(SEM x24,000) Copyright Dr
Dennis
Kunkel, University of Hawaii. Used with permission
|
CORYNEBACTERIA Corynebacterium diphtheriae C. diphtheriae grows best under strict aerobic conditions It is Gram positive and pleomorphic. Colonization of the upper respiratory tract (pharynx and nose) and less commonly skin with C. diphtheriae can lead to diphtheria. The organism does not produce a systemic infection. However, in addition to a pseudomembrane being formed locally (which can cause choking) systemic and fatal injury results primarily from circulation of the potent exotoxin (diphtheria toxin). The latter begins over a period of a week. Thus treatment involves rapid therapy with anti-toxin. The gene for toxin synthesis is encoded on a bacteriophage (the tox gene). Corynebacteria, not infected with phage, thus do not generally cause diphtheria. Diphtheria is now a disease of almost historic importance in the U.S. due to effective immunization of infants (in conjunction with pertussis and tetanus, DPT) with a toxoid (inactive toxin) which causes production of neutralizing antibodies. However, colonization is not inhibited and thus C. diphtheriae is still found in the normal flora (i.e. a carrier state exists). Immunity can be monitored with the Schick skin test. Treatment in non-immune individuals primarily involves injection of anti-toxin. Antibiotics are also administered at this time. The toxin consists of two types of polypeptide. One binds to host cells; the other then becomes internalized and inhibits protein synthesis. The exotoxin catalyses the covalent attachment of the ADP-ribose moiety of NADH to a rare amino acid, diphthamide, present in EF2 (elongation factor 2). The toxin is not synthesized in the presence of iron as an iron-repressor complex forms which inhibits expression of the tox gene. C. diphtheriae are identified by growth on Loeffler's medium followed by staining for metachromatic bodies (polyphosphate granules, Babes-Ernst bodies). The term "metachromatic" refers to the color difference of the intracellular polyphosphate granules (pink) compared to the rest of the cell (blue). Characteristic black colonies are seen on tellurite agar from precipitation of tellurium on reduction by the bacteria. Production of exotoxin can be determined by in vivo or in vitro tests. Other organisms which morphologically resemble C. diphtheriae ("diphtheroids" which include other corynebacteria and also propionibacteria) are found in the normal flora. Isolates should not be confused with these organisms.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
WEB RESOURCES CDC Manual: Diphtheria (pdf) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Legionella pneumophila multiplying inside a cultured
cell. Multiple intracellular bacilli, including dividing bacilli, are visible in longitudinal and cross section. Transmission electron micrograph.
CDC/Dr. Edwin P. Ewing, Jr.
Legionella pneumophila multiplying inside a cultured
cell. Multiple intracellular bacilli, including dividing bacilli, are visible in longitudinal and cross section. Transmission electron micrograph.
CDC/Dr. Edwin P. Ewing, Jr.
|
LEGIONELLAE
In 1976 / 77, Legionella pneumophila was recognized as a newly described pathogen after an investigation by the Centers for Disease Control (CDC) of an outbreak of pneumonia, with several fatalities, among a group of Legionnaires at a convention. The disease was subsequently referred to as Legionnaire's disease. Another milder self-limiting form of the disease (primarily characterized by myalgia [muscle pain]) but no pneumonia is referred to as Pontiac fever. Quickly L. pneumophila filled the world stage as a major pathogen but as rapidly as interest appeared, it waned within the next 10 years, probably because legionellosis was found to be treatable with erythromycin. Occasional small epidemics of Legionnaire's disease in apparently healthy people still occur. However, the organism is more frequently observed in sick or elderly individuals whose immune responses may be compromised. This particularly occurs in the hospital setting. The causative agent was not recognized previously since it does not grow on conventional agar such as sheep blood agar. Nowadays L. pneumophila is cultured successfully on a medium which contains iron and cysteine, which are vital for growth. However, primary isolation is still difficult from clinical specimens. The organism stains poorly as a Gram negative rod. Some legionellae (including L. micdaei) also stain slightly acid fast. However, they are genetically related to other Gram negative rods and NOT mycobacteria. Legionella pneumophila is an organism that resides in the environment in pools of stagnant water. It is found as an intracellular agent within protozoa. In man, it also survives as a facultative intracellular pathogen. It often infects hot water towers and air conditioning systems. Indeed, it is the only bacterial agent whose presence is of major concern in indoor air quality. When found in buildings, cleaning of the water supply is recommended. The organism is transmitted in contaminated air but not spread person-person. After recognition of their unique culture characteristics, a large number of other Legionella-like organisms were isolated from environmental and clinical samples. It should be emphasized these organisms are only occasional causes of human disease and the vast bulk of legionellosis is caused by Legionella pneumophila (most are serogroup 1). The second most common is Legionella (Tatlockia) micdadei The "hot" period of study of these organisms was when it was no longer popular to study bacteria using conventional physiological means. The techniques of fatty acid profiling and DNA-DNA hybridization had just been introduced and were in wide use at the CDC and elsewhere. The new agents were found to be closely related to Legionella pneumophila. Branched fatty acids are characteristic. The CDC thus referred to all species as members of the genus Legionella. Others have broken the family Legionellaceae into three genera: Legionella, Tatlockia and Fluoribacter. Other sophisticated techniques including ubiquinone profiling, carbohydrate profiling and 16S rRNA sequence analysis have sorted out much of the taxonomy of this group of organisms. However, the techniques are far too complex for routine use in the clinical laboratory. Clinically, probes have been used to detect L. pneumophila in clinical specimens and PCR tests are commercially available. Another technique detects the Legionella lipopolysaccharide antigen in urine. However, all approaches (including culture) have limited sensitivity in clinical specimens. The true clinical importance of this organism is thus difficult to judge.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
WEB RESOURCES
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
SOME MAJOR EXOTOXINS
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
This page
copyright 2002, The Board of Trustees of the University of South Carolina |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||