Dr Alvin Fox
Medical Microbiology, MBIM 650/720
Suggested reading: Murray, Third edition Chapters 10 and 20

BACTERIOLOGY - LECTURE FIVE
ANTIBIOTICS THAT AFFECT THE CELL ENVELOPE
Sterilization/disinfection/ antisepsis
Antibiotic
Selective toxicity
Bactericidal
Bacteriostatic
Minimal inhibitory concentration (MIC)
Susceptibility testing
Penicillin binding proteins (PBP)
Autolysins
Cycloserine
Bacitracin
Vancomycin
Beta lactam
Penicillins
Cephalosporins
Monobactam
Clavulinic acid
Penicillinase/beta lactamase
Polymyxin B
Resistance
STERILIZATION
Sterilization refers to killing (or removal) of ALL bacteria in a non-selective fashion. For example, autoclaving involves heating liquids (e.g. media) or solids to 121oC under steam pressure. The materials must be heat resistant. Ethylene oxide is sometimes used in hospitals for equipment that cannot be heated. Membrane filters have pores that trap bacteria, but allow drugs and small chemicals to pass through; thus pre-sterilized filters can be used to sterilize delicate solutions. UV light is used for decreasing bacterial levels on surfaces such as in operating rooms; however it is not totally effective. Ionizing radiation is more efficient and can be used for sterilizing instruments and food.
Disinfectants (e.g. phenol-based) can be useful in killing many bacteria on certain instruments, but cannot be used for internal consumption or on skin. Antiseptics (e.g. iodine or 70% alcohol) are used topically (e.g. on skin surfaces) to reduce bacterial load.
ANTIBIOTICS
In contrast, antibiotics are agents that are "selectively" toxic for bacteria (either killing them [bactericidal] or inhibiting their growth [bacteriostatic]) without harm to the patient. They can thus be ingested. By definition, these compounds must act on structures found in bacteria, but not in the host. Antibiotics work most efficiently in conjunction with an active immune system to kill infecting bacteria in the host. After isolation of pure colonies (see Bacteriology lecture 2 [MBIM lecture 26]), the susceptibility of bacterial isolates can be tested to a variety of antibiotics. The minimal inhibitory concentration (MIC) refers to the lowest concentration of an antibiotic that stops visible growth. More simply, the zone of inhibition around a disk impregnated with antibiotic (Kirby-Bauer) is another measure of antibiotic activity.
INHIBITORS OF CELL WALL SYNTHESIS
One major class of antibiotics inhibit the synthesis of peptidoglycan (figure 1). Once cell wall synthesis (involving penicillin binding proteins) is inhibited, enzymatic autolysis of the cell wall can occur. Without the restraining influence of the cell wall the high osmotic pressure inside the cell bursts the inner and/or outer membranes of bacteria. Thus these antibiotics are generally bactericidal. Several mechanisms are involved in inhibition of peptidoglycan synthesis:
(1) The terminal two amino acids of a peptide side chain of peptidoglycan are unusual amino acids (D-alanine as opposed to its isomer L-alanine). The antibiotic cycloserine is an analog of D-alanine and interferes with enzymatic conversion of L-alanine to D-alanine in the cytoplasm. Thus subsequent synthesis of peptidoglycan cannot occur.
(2) The peptidoglycan subunit (containing one
side-chain and an attached peptide to be used in cross-bridge formation) is
passed across the cytoplasmic membrane attached to undecaprenol diphosphate.
After the nascent peptidoglycan monomer leaves the carrier on reaching the cell
wall, the undecaprenol diphosphate is dephosphorylated to its monophosphate form.
Bacitracin inhibits the dephosphorylation reaction and in the absence of
monophosphorylated carrier peptidoglycan subunit synthesis stops (go here
for further information).
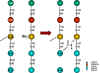 Figure 1 Cross-linking of peptidoglycan
Figure 1 Cross-linking of peptidoglycan
(3) The final step in peptidoglycan synthesis involves linking the sugar portion of the peptidoglycan subunit to the glycan backbone of the existing cell wall polymer. Cross-linking of the peptide portion of the subunit to a peptide in the cell wall then occurs. During this process D-alanine is enzymatically excised from the end of a pre-existing peptide side chain allowing it to be cross-linked (by a new peptide bond) to the recently synthesized peptidoglycan subunit. Vancomycin binds to D-alanine-D-alanine thus sterically inhibits transpeptidation (cross-linking). (go here for further information).
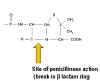
(4) The beta lactam antibiotics include penicillins (e.g. ampicillin), cephalosporins and monobactams. They bind to and inhibit enzymes (penicillin binding proteins) involved in the transpeptidation (cross-linking) of peptidoglycan. These antibiotics have in common the four membered lactam ring. Attached to the lactam, penicillins have an additional five membered ring and cephalosporins a six membered ring. Monobactams consist of the lactam ring alone and display antibiotic activity.
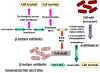 Figure 3 Resistance to beta
lactams - Gram-negative bacteria
Figure 3 Resistance to beta
lactams - Gram-negative bacteria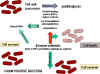 Figure 4 Resistance to beta
lactams - Gram-positive bacteria
Figure 4 Resistance to beta
lactams - Gram-positive bacteriaPENICILLIN
Various chemical side chains have been synthetically linked to the ring structures producing a host of antibiotics with different properties in the host. Many penicillins (figure 2) display little activity against Gram negative bacteria, since they do not penetrate the outer membrane. Cephalosporins and other newer penicillins are active against Gram negative bacteria, since they can penetrate the outer membrane. Other chemically modified penicillins have lower elimination rates from the patient; decreasing the frequency of administration of these drugs.
Penicillins can be destroyed by beta lactamase (penicillinase)
produced by resistant bacterial strains (figure 3). Clavulinic acid, also has a beta lactam
component which binds strongly to beta lactamases inhibiting their activity. It
is used in conjunction with certain penicillins allowing their use against
otherwise resistant bacteria. Another form of resistance involves a change in
the structure of penicillin binding proteins such that the antibiotic does not
bind efficiently (figure 4). In the case of Gram negative bacteria, penicillins pass across
the outer membrane using porins. Resistance may develop from mutation leading to
modified porins.
STREAMING VIDEO
Scale
up - using penicillin as an example
© EBS Trust - From Lifesign
Network
Requires Windows Media Player
 Figure 5 Structure of polymyxin
Figure 5 Structure of polymyxinPOLYMYXIN B
Polymyxin B (figure 5) binds to the lipid A portion of lipopolysaccharide and also to phospholipids. However, it binds preferentially to lipid A. This disrupts the outer membrane of Gram negative bacteria. Since the cell membrane is not exposed in Gram positive bacteria polymyxin has little activity against them. This drug is toxic to human cells, since it can also lyze eukaryotic membranes; this explains its limited clinical use.
![]() Return to the Bacteriology Section of Microbiology and Immunology On-line
Return to the Bacteriology Section of Microbiology and Immunology On-line
![]() Return to the Department of Microbiology and Immunology Site Guide
Return to the Department of Microbiology and Immunology Site Guide
This page
copyright 2000, The Board of Trustees of the University of South Carolina
This page last changed on Wednesday, May 28, 2003
Page maintained by Richard Hunt
URL: http://www.med.sc.edu:85/fox/antibiotics.htm
Please report any problems to rhunt@med.sc.edu