![]()

Dr Abdul Ghaffar
IMMUNOLOGY - CHAPTER TWO
COMPLEMENT
READING: Roitt et al. Immunology (5th ed.),
chapter 4.
Double click on any word to
obtain a definition

TEACHING OBJECTIVES
Understand different pathways of C activation
Know the enzymatic and non-enzymatic mechanisms of complement activation
Know the biological properties of complement activation products
Know the significance of C system in host resistance, inflammation and damage to self
Understand the mechanisms of regulating complement
activation and it products
 Jules Bordet
(1870-1961), discoverer of complement National
Library of Medicine
Jules Bordet
(1870-1961), discoverer of complement National
Library of Medicine
Complement refers, historically, to fresh serum capable of lysing
antibody (Ab)-coated cells. This activity is destroyed (inactivated) by
heating serum at 56 degrees C for 30 minutes.
Proteins of the Complement System
Complement system is composed of more than 25 different proteins (Table 1) produced by different tissues and cells including hepatocytes, macrophages and gut epithelial cells. These proteins are activated by a variety of agents and their activation proceeds in a cascade fashion leading to lysis. Consequently, an absence of one of the components in the pathway can disrupt the cascade and terminate the reaction.
|
Table 1. Proteins of the Complement system |
|||
|
Lytic Pathway |
|||
|
Activation Proteins:
Control Proteins: C1-INH, C4-BP
|
Mannan binding protein (MBP), mannan-asociated serine protease (MASP, MASP2) |
C3, Factors B & D*, Properdin
Factors I* & H, DAF, CR1, etc. |
C5, C6, C7, C8, C9
Protein S |
|
Components underlined acquire enzymatic activity when activated. Components marked with an asterisk have enzymatic activity in their native form. |
|||
Pathways of complement
activation
The complement activation can be divided into three pathways: classical
pathway, alternative pathway and membrane attack pathway. Both
classical and alternative pathways lead to the activation of C5 convertase and
result in the production of C5b which is essential for the activation of the
membrane attack pathway.
Classical pathway
Classical pathway (Figure 1) normally requires a suitable
Ab bound to antigen (Ag), complement components 1, 4, 2 and 3 and Ca++
and Mg++ cations.
MOVIE
Complement Activation and Biological Functions
High Resolution
Quicktime
Low Resolution Quicktime
© Scott R. Barnum, University of Alabama, Birmingham, Ala., USA and The
MicrobeLibrary
C1 activation
Binding of C1qrs (a calcium-dependent complex), present in normal serum, to Ag-Ab complexes results in autocatalysis of C1r. The altered C1r cleaves C1s and this cleaved C1s becomes an enzyme (C4-C2 convertase) capable of cleaving both C4 and C2.
C4 and C2 activation (generation of C3
convertase)
Activated
C1s enzymatically cleaves C4 into C4a and C4b. C4b binds to the Ag-bearing
particle or cell membrane while C4a remains a biologically active peptide
at the reaction site. C4b binds C2 which becomes susceptible to C1s and is
cleaved into C2a and C2b. C2a remains complexed with C4b whereas C2b is released
in the micro environment. C4b2a complex, is known as C3 convertase in
which C2a is the enzymatic moiety.
C3 activation (generation of C5 convertase)
C3
convertase,
in the presence of Mg++, cleaves C3 into C3a and C3b. C3b binds to
the membrane to form C4b2a3b complex whereas C3a remains in the micro
environment. C4b2a3b complex functions as C5 convertase which cleaves C5
into C5a and C5b. Generation of C5 convertase marks the end of the
classical pathway.
Figure 1
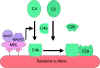 Figure 2 Lectin-initiated pathway
Figure 2 Lectin-initiated pathwayC4 activation can be achieved without antibody and C1 participation by the lectin pathway (Figure 2). This pathway is initiated by three proteins: a mannan-binding lectin (MBL), also known as mannan-binding protein (MBP) which interacts with two mannan-binding lectin-associated serine proteases (MASP and MADSP2), analogous to C1r and C1s. This interaction generates a complex analogous to C1qrs and leads to antibody -independent activation of the classical pathway. C1q can also bind to a number of agents including some retroviruses, mycoplasma, poly-inosinic acid and aggregated IgG, and initiate the classical pathway.
Alternative pathway begins with the activation of C3 and requires Factors B and D and Mg++ cation, all present in normal serum.
Spontaneous activation of C3
A metastable C3b-like molecule (C3i) is generated by slow hydrolysis of the native C3. C3i binds factor B which is cleaved by Factor D to produce C3iBb. C3iBb complex cleaves native C3 into C3a and C3b (Figure 3). C3b binds factor B, which is again cleaved by Factor D to produce C3bBb (C3 convertase). This C3 convertase (or the one generated by classical pathway: C4b2a), if not inactivated, will continue to act on C3 and cause its total depletion.
Normal regulation of C3 convertase
C3b, in fluid phase, is very short lived unless it finds a suitable stabilizing membrane or molecule (C3 activator; see later). In the absence of exogenous pathogen, it binds quickly to autologous red cells via the C3b receptor, CR1 at a site close to decay accelerating factor (DAF) which prevents the binding of Factor B. Binding to CR1 also makes C3b susceptible to Factor I (Figure 4) which cleaves it into many fragments (iC3b, C3d, C3e, etc.). C4b, generated in the classical pathway, is also regulated by DAF, CR1 and Factor I (Figure 5). A defect in or deficiency of DAF can lead to cell lysis and anemia, as in its absence further activation of C will proceed and lead to the membrane attack pathway (see below) and cell lysis.
Another serum protein, factor H, can displace factor B and bind to C3b. Binding of factor H makes C3b more susceptible to factor I (see figure 4). C3 convertase generated by the classical pathway is regulated also in a similar manner by DAF, Cr1 and Factor I. The only difference is that C4b-binding protein (C4b-BP, not factor H) makes it susceptible to Factor I. A genetic deficiency of factor I (or factor H) leads to uncontrolled C3 activation and is a major cause of inherited C3 deficiency.
Certain bacteria or their products (peptidoglycan, polysaccharides, etc.), provide a protected (activator) surface for C3b. Thus, C3b bound to such a surface is relatively resistant to the action of factor I (Figure 6). Even membrane bound C3bBb dissociates fairly rapidly. However, binding of another protein, properdin, further stabilizes this complex. It is for this reason, the alternative pathway is also called the properdin pathway.Stabilization of C3 convertase
Generation of C5 convertase
Stabilized C3 convertase
cleaves more C3 and produces C3bBbC3b complex (analogous to C4b2a3b of the
classical pathway), the C5 convertase which cleaves C5 into C5a and C5b
(Figure 6). C5b initiates the membrane attack pathway which leads to cell lysis. While these pathways of C3 activation are initiated by different
mechanisms, they are analogous to each other and both can lead to membrane lysis.
The alternative pathway provides a means of non-specific resistance against infection without the participation of antibodies and hence provides a first line of defense against a number of infectious agents.
Many gram negative and some gram positive bacteria, certain viruses, parasites, heterologous red cells, aggregated immunoglobulins (particularly, IgA) and some other proteins (e.g. proteases, clotting pathway products) can activate the alternative pathway. One protein, cobra venom factor (CVF), has been extensively studied for its ability to activate this pathway.
The lytic (membrane attack) pathway involves the C5-9 components. C5 convertase generated by the classical or alternative pathway cleaves C5 into C5a and C5b. C5b binds C6 and subsequently C7 to yield a hydrophobic C5b67 complex which attaches quickly to the plasma membrane (Figure 7). Subsequently, C8 binds to this complex and causes the insertion of several C9 molecules. bind to this complex and lead to formation of a hole in the membrane resulting in cell lysis. The lysis of target cell by C5b6789 complex is nonenzymatic and is believed to be due to a physical change in the plasma membrane. C5b67 can bind indiscriminately to any cell membrane leading to cell lysis. Such an indiscriminate damage to by-standing cells is prevented by protein S (vitronectin) which binds to C5b67 complex and blocks its indiscriminate binding to cells other than the primary target.
 Figure 8
Regulation of C1rs (C4 convertase) by C1-INH
Figure 8
Regulation of C1rs (C4 convertase) by C1-INHBiologically active products of Complement activation
Activation of complement results in the production of several biologically active molecules which contribute to resistance, anaphylaxis and inflammation.
Kinin production
C2b generated during the classical pathway
of C activation is a prokinin which becomes biologically active following
enzymatic alteration by plasmin. Excess C2b production is prevented by limiting
C2 activation by C1 inhibitor (C1-INH) also known as serpin which
displaces C1rs from the C1qrs complex (Figure 8). A genetic deficiency of C1-INH
results in an overproduction of C2b and is the cause of hereditary
angioneurotic edema. This condition can be treated with
Danazol which
promotes C1-INH production or with ε-amino
caproic acid which decreases plasmin activity.
WEB RESOURCES
Hereditary angioedema
On-line
Mendelian inheritance in man (NIH)
Anaphylotoxins
C4a, C3a and C5a (in increasing order of
activity) are all Anaphylotoxins which cause basophil/mast cell
degranulation and smooth muscle contraction. Undesirable effects of these
peptides are controlled by carboxypeptidase B (C3a-INA).
Chemotactic Factors
C5a and MAC (C5b67) are both
chemotactic. C5a is also a potent activator of neutrophils, basophils and
macrophages and causes induction of adhesion molecules on vascular
endothelial cells.
Opsonins
C3b and C4b in the surface of microorganisms
attach to C-receptor (CR1) on phagocytic cells and promote phagocytosis.
Other Biologically active products of
C activation
Degradation products of C3 (iC3b, C3d and C3e) also bind to different cells by
distinct receptors and modulate their functions.
In summary, the complement system takes part in both specific and non-specific resistance and generates a number of products of biological and pathophysiological significance (Table 2).
There are known genetic deficiencies of most individual C complement components, but C3 deficiency is most serious and fatal. Complement deficiencies also occur in immune complex diseases (e.g., SLE) and acute and chronic bacterial, viral and parasitic infections.
You have learned
The proteins of the complement system
The differences and similarities among the different pathways of C3 activation
The significance of the different pathways in specific and nonspecific immunity
The role of different complement activation products in amplification of nonspecific and specific immunity and inflammation
|
Table 2: Biological Properties of C Activation Products and their Regulatory Molecules |
|||
|
Component |
Biological activity |
Effect |
Controls |
| C2b (prokinin) | Accumulation of body fluid |
Edema |
C1-INH |
| C3a (anaphylatoxin) |
Basophil and mast cell degranulation; enhanced vascular permeability; smooth muscle contraction |
Anaphylaxis | Carboxy-peptidase- B (C3a-INA) |
| Induction of suppressor T cells. | Immunoregulation | ||
| C3b and its products | Opsonization; Phagocyte activation |
Phagocytosis |
Factors H & I |
| C4a (anaphylatoxin) | Basophil & mast cell activation; smooth muscle contraction; enhanced vascular permeability. | Anaphylaxis | C3a-INA |
| C4b | Opsonization | Phagocytosis | C4-BP, Factor I |
|
C5a (anaphylatoxin; Chemotactic factor) |
Basophil & mast cell activation; enhanced vascular permeability; smooth muscle contraction. | Anaphylaxis | C3a INA |
| Chemotaxis; neutrophil aggregation; Oxidative metabolism stimulation. | Inflammation | ||
| Stimulation of leukotriene release | Delayed anaphylaxis. | ||
| Induction of helper T-cells. | Immunoregulation. | ||
| C5b67 | Chemotaxis; attachment to other cell membranes. |
Inflammation; lysis of bystander cells. |
Protein-S |

![]() Return to the Department of Microbiology and Immunology Site Guide
Return to the Department of Microbiology and Immunology Site Guide
![]() Return to the Immunology Section of Microbiology and Immunology On-line
Return to the Immunology Section of Microbiology and Immunology On-line
This page copyright
2004, The Board of
Trustees of the University of South Carolina
This page last changed on
Friday, November 04, 2005
Page maintained by Richard Hunt
URL: http://www.med.sc.edu:85/ghaffar/complement.htm
Please report any problems to rhunt@med.sc.edu