|
x |
x |
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
|
PARASITOLOGY - CHAPTER TWO
BLOOD AND TISSUE PROTOZOA
Dr
Abdul
Ghaffar
Professor Emeritus
University of South Carolina
|
|
|
|
|
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|

 |
|
|
|
|
|
|
Blood protozoa of
major clinical significance include members of genera:
-
Trypanosoma (T. brucei and
T. cruzi)
-
Leishmania (L. donovani,
L. tropica and L.
braziliensis)
-
Plasmodium (P. falciparum,
P. ovale, P. malariae and
P. vivax)
-
Toxoplasma gondii
-
Babesia (B. microti)
TRYPANOSOMIASIS
African
trypanosomiasis (Sleeping sickness)
Etiology
There are
two clinical forms of African trypanosomiasis:
-
A slowly developing disease,
West African Sleeping Sickness, caused by Trypanosoma brucei
gambiense
-
A rapidly progressing disease,
East African Sleeping Sickness, caused by T. brucei
rhodesiense.
Epidemiology
T. b.
gambiense is predominant in the western and central regions of Africa, whereas
T. b. rhodesiense is restricted to the eastern third of the continent
(figure 2E).
Each year, there are only a few
hundred cases of East African Sleeping Sickness with almost all being
occurring in Zambia, Malawi, Uganda and Tanzania; however, 6,000 to
10,000 human cases of West African Sleeping Sickness are documented annually
with most cases occurring in The Central African Republic, The Democratic
Republic of the Congo, northern Uganda, Chad, Angola and Sudan. Thirty five million people and 25
million cattle are at risk. Regional epidemics of the disease are cause of major
health and economic disasters.
Occasionally, a traveler to
endemic counties contracts Sleeping Sickness. About one case of East African
Sleeping Sickness is imported into the United States each year, usually in
someone who has recently travelled to the region. In the case, of West
African Sleeping Sickness, most infections diagnosed in the United States
are in people who have immigrated from an endemic region. These are very
rare.
Vector and Reservoir
In both West African and East African Sleeping Sickness, the vector is
the Tsetse Fly (Glossina sp) and both sexes of the fly can transmit
the parasite in their saliva. In endemic areas, however, only a few flies
are carrieers. The animal reservoir for T. b.
gambiense is other humans but domestic animals can also carry the parasite.
The reservoirs for T. b. rhodesiense are wild animals and cattle.
Very occasionally, an unborn
baby may be infected from an infected mother. It is also possible that
people have been very rarely infected as a result of blood transfusions.
Morphology
T. b.
gambiense and T. b. rhodesiense are similar in appearance: The
organism measures 10 - 30 micrometers x 1-3 micrometers. It has a single central nucleus and a single flagellum originating at the kinetoplast and
joined to the body by an undulating membrane (Figure 2A-D). The outer surface of
the organism is densely coated with a layer of glycoprotein, the variable
surface glycoprotein (VSG).
|
|
TEACHING
OBJECTIVES
Epidemiology,
morbidity and mortality
Morphology of the organism
Life
cycle, hosts and vectors
Disease,
symptoms, pathogenesis and site
Diagnosis
Prevention
and control
|
|
|
|
|
 Figure 1A
Figure 1A
During a blood meal on the mammalian host, an infected tsetse fly
(genus Glossina) injects metacyclic trypomastigotes into skin tissue.
The parasites enter the lymphatic system and pass into the bloodstream
 .
Inside the host, they transform into bloodstream trypomastigotes .
Inside the host, they transform into bloodstream trypomastigotes
 ,
are carried to other sites throughout the body, reach other blood fluids (e.g.,
lymph, spinal fluid), and continue the replication by binary fission ,
are carried to other sites throughout the body, reach other blood fluids (e.g.,
lymph, spinal fluid), and continue the replication by binary fission
 .
The entire life cycle of African Trypanosomes is represented by extracellular
stages. The tsetse fly becomes infected with bloodstream trypomastigotes
when taking a blood meal on an infected mammalian host ( .
The entire life cycle of African Trypanosomes is represented by extracellular
stages. The tsetse fly becomes infected with bloodstream trypomastigotes
when taking a blood meal on an infected mammalian host ( , ,
 ). In the fly’s midgut, the
parasites transform into procyclic trypomastigotes, multiply by binary fission ). In the fly’s midgut, the
parasites transform into procyclic trypomastigotes, multiply by binary fission
 ,
leave the midgut, and transform into epimastigotes ,
leave the midgut, and transform into epimastigotes
 .
The epimastigotes reach the fly’s salivary glands and continue multiplication
by binary fission .
The epimastigotes reach the fly’s salivary glands and continue multiplication
by binary fission  . The cycle
in the fly takes approximately 3 weeks. Humans are the main reservoir for Trypanosoma
brucei gambiense, but this species can also be found in animals. Wild
game animals are the main reservoir of T. b. rhodesiense. . The cycle
in the fly takes approximately 3 weeks. Humans are the main reservoir for Trypanosoma
brucei gambiense, but this species can also be found in animals. Wild
game animals are the main reservoir of T. b. rhodesiense.
CDC DPDx Parasite Image Library
 Figure 1B Forms of Trypansoma brucei obsreved in the tstese
fly and in the human blood stream
Figure 1B Forms of Trypansoma brucei obsreved in the tstese
fly and in the human blood stream
T. brucei is transmitted by tsetse flies of the genus Glossina.
Parasites are ingested by the fly when it takes a blood meal on an infected
mammal. The parasites multiply in the fly, going through several developmental
stages in the insect gut and salivary glands (procyclic trypanosomes,
epimastigotes, metacyclic trypanosomes). The cycle in the fly takes
approximately 3 weeks. When the fly bites another mammal, metacyclic
trypanosomes are inoculated, and multiply in the host's blood and extracellular
fluids such as spinal fluid. Humans are the main reservoir for T. b. gambiense,
but this species can also be found in animals. Wild game animals are the main
reservoir of T. b. rhodesiense.

 Figure 2A
Figure 2A
Two areas from a blood smear from a patient with African trypanosomiasis. Thin blood smear stained with Giemsa. Typical
trypomastigote stages (the only stages found in patients), with a
posterior kinetoplast, a centrally located nucleus, an undulating
membrane, and an anterior flagellum. The two Trypanosoma brucei species
that cause human trypanosomiasis, T. b. gambiense and
T. b. rhodesiense, are undistinguishable morphologically. The
trypanosomes length range is 14-33 µm
CDC DPDx Parasite Image Library
 Figure 2B
Figure 2B
Blood smear from a patient (a U.S. traveler) with Trypanosoma
brucei rhodesiense. A dividing parasite is seen at the right. Dividing
forms are seen in African trypanosomiasis, but not in American
trypanosomiasis (Chagas' disease)
CDC DPDx Parasite Image Library
 Figure 2D Figure 2D
Structure of Trypanosome brucei
 Figure 2E
Figure 2E
Distribution of West African or Gambian Sleeping Sickness and East
African or Rhodesian Sleeping Sickness
 Figure 2C Figure 2C
Blood smear from a patient with Trypanosoma brucei gambiense.
CDC -
Image contributed by Pr. J. Le Bras, Hôpital Bichat - Claude Bernard, Paris,
France.
 Figure 2F Reported number of cases of African
trypanosomiasis
Figure 2F Reported number of cases of African
trypanosomiasis
in Uganda, 1939-1998 WHO
Between 1962 and 1975, no cases were reported. Increased reporting
during 1977 to 1983 reflected an epidemic of rhodesiense sleeping
sickness in Busuga (south-eastern Uganda). However the increases shown
between 1986 and 1992 corresponded to both the resumption of systematic
population screening for gambiense sleeping sickness in the western part
of the country and to a resurgence of rhodesiense sleeping sickness in
Busuga.
|
| |
 Figure 3 Tsetse fly. The vector of African trypanosomiasis
© OhioState University, College of Biology
Figure 3 Tsetse fly. The vector of African trypanosomiasis
© OhioState University, College of Biology |
Life cycle
The
infective, metacyclic form of the trypanosome is injected into the primary host
during a bite by the vector, the tsetse fly (figure 3). The organism transforms
into a dividing trypanosomal (trypomastigote) blood form (figure 1B) as it enters the
draining lymphatic and blood stream. The trypanosomal form enters the vector
during the blood meal and travels through the alimentary canal to the salivary
gland where it proliferates as the crithidial form (epimastigote) and matures to
infectious metacyclic forms (Figure 1B). Trypomastigotes can traverse the walls
of blood and lymph capillaries into the connective tissues and, at a later
stage, cross the choroid plexus into the brain and cerebrospinal fluid. The
organism can be transmitted through blood transfusion.
|
| |
Symptoms
The clinical
features of Gambian and Rhodesian disease are the same, however they vary in
severity and duration. Rhodesian disease progresses more rapidly and the
symptoms are often more pronounced. The symptoms of the two diseases are also
more pronounced in Caucasians than in the local African population.
Classically, the progression of African trypanosomiasis can be divided into
three stages: the bite reaction (chancre), parasitemia (blood and lymphoid
tissues), and CNS stage.
Bite reaction
A
non-pustular, painful, itchy chancre (Figure 4 A and B) forms 1-3 weeks after the
bite and lasts 1-2 weeks. It leaves no scar.
Parasitemia
Parasitemia and lymph node invasion is marked by attacks of fever which
starts 2-3 weeks after the bite and is accompanied by malaise, lassitude,
insomnia headache and lymphadenopathy and edema (figure 4E). Painful
sensitivity of palms and ulnar region to pressure (Kerandel's sign) may
develop in some Caucasians. Very characteristic of Gambian disease is
visible enlargement of the glands of the posterior cervical region (Winterbottom's
sign) (Figure 4C). Febrile episodes may last few months as in Rhodesian
disease or several years as in Gambian disease. Parasitemia is more
prominent during the acute stage than during the recurrence episodes.
CNS Stage
The
late or CNS stage is marked by changes in character and personality. They
include lack of interest and disinclination to work, avoidance of
acquaintances, morose and melancholic attitude alternating with exaltation,
mental retardation and lethargy, low and tremulous speech, tremors of tongue
and limbs, slow and shuffling gait, altered reflexes, etc. Males become
impotent. There is a slow progressive involvement of cardiac tissue. The
later stages are characterized by drowsiness and uncontrollable urge to
sleep. The terminal stage is marked by wasting and emaciation. Death results
from coma,
intercurrent infection or cardiac failure (figure 5).
In the case of T. b. rhodesiense
disease, death occurs within months of CNS involvement whereas T. b.
gambiense-caused disease is slower and, without treatment, death occurs
within 3 to 7 years.
|
|
Figure
4A
 The partially healed chancre on the arm of a female patient in a ward of a rural clinic.
WHO/TDR/Crump
The partially healed chancre on the arm of a female patient in a ward of a rural clinic.
WHO/TDR/Crump |
 Figure
4B
Figure
4B
The leg of a teenage girl who has sleeping sickness, showing the chancre at the site of the tsetse fly bite
WHO/TDR/Kuzoe
 Figure
4C
Figure
4C
Winterbottoms sign CDC
DPDx Parasite Image Library
 Figure
4D
Figure
4D
Neurological complications can occur as a result of infection and, as seen here, patients may be immobilised for their own safety.
WHO/TDR/Kuzoe
 Figure
4E
Figure
4E
A male sleeping sickness patient with myxoedema.
WHO/TDR/Kuzoe
 Figure 5A
Figure 5A
The damaged brain of a patient who had died from African trypanosomiasis (or sleeping sickness).
WHO/TDR/Kuzoe
 Figure 5B
Figure 5B
A young boy with advanced African trypanosomiasis (or sleeping sickness) exhibiting marked wasting and skin damage caused as a result of the intense itching which can accompany late-stage disease.
WHO/TDR/Kuzoe
 Figure 5C
Figure 5C
Neuropathology of Human African Trypanosomiasis: Acute haemorrhagic leucoencephalopathy
(AHL): This slide shows very delicate fibrinoid necrosis in the wall of a small artery in the thalamus.
Produced by the Dept. of
Neuropathology, Southern General Hospital,
Glasgow).
 Figure 5D
Figure 5D
Neuropathology of Human African Trypanosomiasis: Acute haemorrhagic
leucoencephalopathy: This slide shows the foci of haemorrhage around small blood vessels.
Produced by the Dept. of
Neuropathology, Southern General Hospital, Glasgow).
|
|
|
| |
The clinical features
of Rhodesian disease are similar but briefer and more acute. The acuteness and
severity of disease do not allow typical sleeping sickness. Death is due to
cardiac failure within 6-9 months.
Pathology and
Immunology
An exact pathogenesis of
sleeping sickness is not known, although
immune complexes and inflammation have been suspected to be the mechanism of
damage to tissues. The immune response against the organism does help to eliminate the
parasite but it is not protective, since the parasite has a unique ability of
altering its surface antigens, the Variable Surface Glycoproteins (VSGs) - see the chapter on
Molecular
Biology of Trypanosomes. Consequently, there is a cyclic fluctuation in
the number of parasites in blood and lymphatic fluids and each wave of parasite
represents a different antigenic variant. The parasite causes polyclonal
expansion of B lymphocytes and plasma cells and an increase in total IgM
concentration. It stimulates the reticuloendothelial function. It also causes
severe depression of cell mediated and humoral immunity to other antigens.
Diagnosis
Detection
of parasite by microscopy in the bloodstream, lymph secretions and enlarged lymph node aspirate
provides a definitive diagnosis in early (acute) stages. Classically, a lymph
node (posterior cervical node) aspirate is used as it may be difficult to
detect a low
parasitemia in the blood. The parasite in blood can
be concentrated by centrifugation or by the use of anionic support media.
Cerebrospinal fluid must always be examined for organisms. Immuno-serology (enzyme-linked immune assay, immunofluorescence) may
be indicative but does not provide definite diagnosis.
Treatment and
Control
The blood stage of African trypanosomiasis can be treated with reasonable
success according to the stage that the disease has reached. Pentamidine isethionate
is used for first stage T. b. gambiense infection. Other drugs
available for use are
suramin,
melarsoprol, eflornithine or nifurtimox. Suramin has been reported
also to be effective in prophylaxis although they may mask early infection and
thus increase the risk of CNS disease. Cases with CNS involvement should be
treated with melarsoprol, an organic arsenic compound; however this drug has
been linked to fatal encephalopathy.
The most effective
means of prevention is to avoid contact with tsetse flies. Vector eradication is
usually impractical due to the vast area involved. Immunization has not been effective
due to antigenic variation.
|
 Figure 6 Chaga's disease: Countries in which American trypanosomiasis is endemic.
WHO
Figure 6 Chaga's disease: Countries in which American trypanosomiasis is endemic.
WHO |
American
trypanosomiasis (Chagas' disease)
Etiology
Chagas'
disease is caused by the protozoan hemoflagellate, Trypanosoma cruzi.
Epidemiology
American
trypanosomiasis, also known as Chagas' disease, is scattered irregularly in
Central and South America, stretching from parts of Mexico to Argentina (figure
6). It is estimated that over 8 million people are infected by the parasite and
50 million are at risk. About 50,000 people die each year from the disease.
CDC estimates that there are as
many as 300,000 infected people in the United States and cases
cases have been reported in Texas, California and Maryland. Most of these
infections were acquired in countries of Central and South America where the
disease is endemic and vector-borne cases are very rare..
|
 Figure 7A
Figure 7A
Trypanosoma cruzi, trypomastigote form, in a blood smear (Giemsa stain)
CDC
DPDx Parasite Image Library |
Morphology
Depending
on its host environment, the organism occurs in three different forms (Figure 7
and 9B).
- The trypanosomal (trypomastigote) form (figure 7A), found in mammalian blood, is 15 to 20
microns long and morphologically similar to African trypanosomes.
- The crithidial
(epimastigote) form (figure 7B) is found in the insect intestine.
- The leishmanial
(amastigote)
form (figure 7C), found intracellularly or in pseudocysts in mammalian viscera
(particularly in myocardium and brain), is round or oval in shape, measures 2-4
microns and lacks a prominent flagellum.
|

Figure 7B Trypanosoma cruzi, crithidia.
CDC
DPDx Parasite Image Library |
Life
cycle
The
organism is transmitted to mammalian host by many species of kissing or
triatomine (riduvid)
bug (figure 8), most prominently by Triatoma infestans, Triatoma sordida,
Panstrongylus megistus
and Rhodnius prolixus.Transmission takes place during the feeding of the bug
which normally bites in the facial area (hence the name, kissing bug) and has
the habit of defecating during feeding. The metacyclic trypamastigotes, contained
in the fecal material, gain access to the mammalian tissue through the wound
which is often rubbed by the individual that is bitten. Subsequently,
they enter various cells, including macrophages, where they
differentiate into amastigotes and multiply by binary fission. The
amastigotes differentiate into non-replicating trypomastigotes and the
cells rupture to release them into the bloodstream. Additional host
cells, of a variety of types, can become infected and the
trypomastigotes once again form amastigotes inside these cells. Uninfected
insect vectors acquire the organism when they
feed on infected animals or people containing trypomastigotes circulating in
their blood. Inside the alimentary tract of the insect vector, the trypomastigotes
differentiate to form epimastigotes and divide longitudinally in the mid
and hindgut of the insect where they develop into infective metacyclic
trypomastigotes (figure 9C).
Transmission may also occur
between humans by
- Blood
transfusion
- Mother to baby via a transplacental route
- Organ transplantation
- Very rarely via contaminated
food or drink
|

Figure 7C. Trypanosoma cruzi. Leishmanial form CDC
DPDx Parasite Image Library
 Figure 8 Riduvid bug, the vector of American trypanosomiasis
Figure 8 Riduvid bug, the vector of American trypanosomiasis
 Figure 9A Ramana's sign: unilateral
conjunctivitis and orbital edema
Figure 9A Ramana's sign: unilateral
conjunctivitis and orbital edema
 Figure 9B Megacolon in Chaga's disease
Figure 9B Megacolon in Chaga's disease |
More than one hundred mammalian species of wild and domestic animals including cattle, pigs, cats,
dogs, rats, armadillo, raccoon and opossum are naturally infected by T. cruzi
and serve as a reservoir.
Symptoms
Chagas'
disease can be divided into three stages: the primary lesion, the acute stage,
and the chronic stage. The primary lesion, chagoma, appearing at the site of
infection, within a few hours of a bite, consists of a slightly raised, flat
non-purulent erythematous plaque surrounded by a variable area of hard edema. It
is usually found on the face, eyelids, cheek, lips or the conjunctiva, but may
occur on the abdomen or limbs. When the primary chagoma is on the face, there is
an enlargement of the pre- and post- auricular and the submaxillary glands on
the side of the bite. Infection in the eyelid, resulting in a unilateral
conjunctivitis and orbital edema (Ramana's sign) (figure 9A), is the commonest finding.
Acute Stage: The
acute stage appears 7-14 days after infection. It is characterized by
restlessness, sleeplessness, malaise, increasing exhaustion, chills, fever
and bone and muscle pains. Other manifestations of the acute phase are
cervical, axillary and iliac
adenitis,
hepatomegaly,
erythematous rash and
acute
myocarditis. There is a general edematous reaction associated with
lymphadenopathy. Diffuse myocarditis, sometimes accompanied by serious
pericarditis and endocarditis, is very frequent during the initial stage of
the disease. In children, Chagas' disease may cause meningo-encephalitis and coma. Death
occurs in 5-10 percent of infants. Hematologic examination reveals
lymphocytosis and parasitemia.
Chronic Stage: The
acute stage is usually not recognized and often resolves with little or no
immediate damage and the infected host remains an asymptomatic carrier. An
unknown proportion (guessed at 10-20%) of victims develop a chronic disease.
They alternate between asymptomatic remission periods and relapses
characterized by symptoms seen in the acute phase. Cardiac arrhythmia is
common. The chronic disease results in an abnormal function of the hollow
organs, particularly the heart, esophagus and colon.
The cardiac changes
include myocardial insufficiency, cardiomegaly, disturbances of atrio-ventricular
conduction and the Adams-Stoke syndrome. Disturbances of peristalsis lead to
megaesophagus and megacolon (figure 9B).
|
|
|
Pathology and
Immunology
The pathological effects of acute phase
Chagas' disease largely result from
direct damage to infected cells. In later stages, the destruction of the autonomic
nerve ganglions may be of significance. Immune mechanisms, both cell mediated
and humoral, involving reaction to the organism and to autologous tissues have
been implicated in pathogenesis.
T. cruzi stimulates
both humoral and cell mediated immune responses. Antibody has been shown to lyze
the organism, but rarely causes eradication of the organism, perhaps due to its
intracellular localization. Cell mediated immunity may be of significant value.
While normal macrophages are targeted by the organism for growth, activated
macrophages can kill the organism. Unlike T. brucei, T. cruzi does not alter its
antigenic coat. Antibodies directed against heart and muscle cells have also
been detected in infected patients leading to the supposition that there is an element of autoimmune reaction in the
pathogenesis of Chagas' disease. The infection causes severe depression of both
cell mediated and humoral immune responses. Immunosuppression may be due to
induction of suppressor T-cells and/or overstimulation of macrophages.
Diagnosis
Clinical
diagnosis is usually easy among children in endemic areas. Cardiac dilation,
megacolon and megaesophagus in individuals from endemic areas indicate present
or former infection. Definitive diagnosis requires the demonstration of
trypanosomes by microscopy or biological tests (in the insect or mice). Antibodies
are often detectable by complement fixation or immunofluorescence and provide
presumptive diagnosis.
Treatment and
Control
There is no curative therapy available. Most drugs are either ineffective or
highly toxic. Recently two experimental drugs, Benznidazol and Nifurtimox have
been used with promising results in the acute stage of the disease, however
their side effects limit their prolonged use in chronic cases.
Control measures are
limited to those that reduce contact between the vectors and man. Attempts to
develop a vaccine have not been very successful, although they may be feasible.
|
| |
|
|
 Figure 9C
Figure 9C
An infected triatomine insect vector (or “kissing” bug) takes
a blood meal and releases trypomastigotes in its feces near the site of the bite
wound. Trypomastigotes enter the host through the wound or through intact
mucosal membranes, such as the conjunctiva
 .
Common triatomine vector species for trypanosomiasis belong to the genera Triatoma,
Rhodinius, and Panstrongylus. Inside the host, the
trypomastigotes invade cells, where they differentiate into intracellular
amastigotes .
Common triatomine vector species for trypanosomiasis belong to the genera Triatoma,
Rhodinius, and Panstrongylus. Inside the host, the
trypomastigotes invade cells, where they differentiate into intracellular
amastigotes  . The amastigotes
multiply by binary fission . The amastigotes
multiply by binary fission  and
differentiate into trypomastigotes, and then are released into the circulation
as bloodstream trypomastigotes and
differentiate into trypomastigotes, and then are released into the circulation
as bloodstream trypomastigotes  .
Trypomastigotes infect cells from a variety of tissues and transform into
intracellular amastigotes in new infection sites. Clinical manifestations
can result from this infective cycle. The bloodstream trypomastigotes do
not replicate (different from the African trypanosomes). Replication
resumes only when the parasites enter another cell or are ingested by another
vector. The “kissing” bug becomes infected by feeding on human or
animal blood that contains circulating parasites .
Trypomastigotes infect cells from a variety of tissues and transform into
intracellular amastigotes in new infection sites. Clinical manifestations
can result from this infective cycle. The bloodstream trypomastigotes do
not replicate (different from the African trypanosomes). Replication
resumes only when the parasites enter another cell or are ingested by another
vector. The “kissing” bug becomes infected by feeding on human or
animal blood that contains circulating parasites
 .
The ingested trypomastigotes transform into epimastigotes in the vector’s
midgut .
The ingested trypomastigotes transform into epimastigotes in the vector’s
midgut  . The parasites
multiply and differentiate in the midgut . The parasites
multiply and differentiate in the midgut
 and differentiate into infective metacyclic trypomastigotes in the hindgut
and differentiate into infective metacyclic trypomastigotes in the hindgut
 . .
Trypanosoma cruzi can also be transmitted through blood transfusions,
organ transplantation, transplacentally, and in laboratory accidents.
CDC
DPDx Parasite Image Library
|
|
Guest article
New approaches
for vaccines against a neglected disease – leishmaniasis |
LEISHMANIASIS
Etiology
More than 20
species of Leishmania are pathogenic for man:
-
L. donovani causes visceral
leishmaniasis (Kala-azar, black disease, dumdum fever)
-
L. tropica
(L. t.
major, L. t. minor and L. ethiopica) causes cutaneous leishmaniasis (oriental
sore, Delhi ulcer, Aleppo, Delhi or Baghdad boil)
-
L. braziliensis
(also, L. mexicana and L. peruviana) are etiologic agents of mucocutaneous
leishmaniasis (espundia, Uta, chiclero ulcer)
Epidemiology
Leishmaniasis is prevalent
in more than 90 countries world wide: ranging from south east Asia,
Indo-Pakistan, Mediterranean area of southern Europe, north and central Africa, and south and central
America. A few cases of cutaneous leishmaniasis have been found in the United
States (Texas and Oklahoma). These have been acquired during travel to
endemic areas
The annual number of cases
worldwide have been estimated to be:
-
Cutaneous leishmaniasis:
Between 700,000 and 1.2 million
-
Visceral leishmaniasis:
Between 200,000 and 400,000
Morphology
Amastigote
(leishmanial form) is oval and measures 2-5 microns by 1 - 3 microns (figure
10A-D), whereas the
leptomonad measures 14 - 20 microns by 1.5 - 4 microns, a similar size to trypanosomes
(Figure 10E).
|
|
|
|
Figure
10 A B C
A
 B
B
 C
C
 Leishmania tropica amastigotes from a skin touch preparation. In A, a
still intact macrophage is practically filled with amastigotes, several
of which have clearly visible a nucleus and a kinetoplast (arrows); in
B, amastigotes are being freed from a rupturing macrophage. Patient with
history of travel to Egypt, Africa, and the Middle East. Culture in NNN
medium followed by isoenzyme analysis identified the species as L.
tropica minor. CDC
Leishmania tropica amastigotes from a skin touch preparation. In A, a
still intact macrophage is practically filled with amastigotes, several
of which have clearly visible a nucleus and a kinetoplast (arrows); in
B, amastigotes are being freed from a rupturing macrophage. Patient with
history of travel to Egypt, Africa, and the Middle East. Culture in NNN
medium followed by isoenzyme analysis identified the species as L.
tropica minor. CDC |
 Figure 10D
Figure 10D
Leishmania mexicana mexicana in skin biopsy. Hematoxylin and eosin stain. The amastigotes are lining the wall of two
vacuoles, a typical arrangement. The species identification was derived from culture followed by isoenzyme analysis. 26-year old man from Austin, Texas, with a lesion on his left arm.
CDC DPDx Parasite Image Library
 Figure 10E
Figure 10E
Leishmania donovani, leptomonad forms.
CDC
DPDx Parasite Image Library
 Figure 10G Figure 10G
Bone marrow smear showing Leishmania donovani parasites in a bone marrow histiocyte from a dog
(Giemsa stain).
CDC/Dr. Francis W. Chandler
 Figure 10I
Figure 10I
Leishmania donovani in bone marrow cell. Smear.
CDC/Dr. L.L. Moore, Jr.
 Figure 10 F
Figure 10 F
Giemsa stained
leishmanial promastigotes from a culture in which the bar-shaped kinetoplast in the organism closest to the center of the group "rosette" may be
seen.
©
Lynne S. Garcia,
LSG & Associates, Santa Monica, California
and Microbe Library
 Figure 10H
Figure 10H
Erythrophagocytosis in the liver (H&E X 400)
WHO/TDR/El-Hassan
 Figure 10J
Figure 10J
Periarterial sheath of macrophages of the spleen showing
heavy parasitisation with amastigotes (H&E X 400)
WHO/TDR/El-Hassan
|
| |
Life cycle
The
organism is transmitted by the bite of about 30 species of blood-feeding sand
flies (Phlebotomus) which carry the promastigote in the anterior gut and
pharynx. The parasites gain access to mononuclear phagocytes where they transform into
amastigotes and divide until the infected cell ruptures. The released organisms
infect other cells. The sandfly acquires the organisms during the blood meal;
the amastigotes transform into flagellate promastigotes and multiply in the gut
until the anterior gut and pharynx are packed. Dogs and rodents are
common reservoirs (figure 11F).
Symptoms
Visceral
leishmaniasis (kala-azar, dumdum fever): L. donovani organisms in visceral
leishmaniasis are rapidly eliminated from the site of infection, hence there
is rarely a local lesion, although minute papules have been described in
children. They are localized and multiply in the mononuclear phagocytic cells
of spleen, liver, lymph nodes, bone marrow, intestinal mucosa and other
organs. One to four months after infection, there is occurrence of fever, with a
daily rise to 102-104 degrees F, accompanied by chills and sweating.
The spleen
and liver progressively become enlarged (figure 11B, C and E). With progression of the diseases,
skin develops hyperpigmented granulomatous areas (kala-azar means black disease).
Chronic disease renders patients susceptible to other infections. Untreated
disease results in death.
|

Figure 11A
Many children suffering from visceral leishmaniasis develop a noticeable thickening, stiffening and darkening of the eyelashes and eyebrows.
WHO/TDR/Crump |
 Figure 11B
Figure 11B
Profile view of a teenage boy suffering from visceral
leishmaniasis. The
boy exhibits splenomegaly, distended abdomen and severe muscle wasting.
WHO/TDR/Kuzoe
 Figure 11C
Figure 11C
A 12-year-old boy suffering from visceral
leishmaniasis. The boy exhibits
splenomegaly and severe muscle wasting.
WHO/TDR/El-Hassan
 Figure 11D
Figure 11D
Jaundiced hands of a visceral leishmaniasis patient.
WHO/TDR/El-Hassan
 Figure 11E Figure 11E
Enlarged spleen and liver in an autopsy of an infant dying of visceral
leishmaniasis.
WHO/TDR/El-Hassan
 Figure 11F
Figure 11F
Leishmaniasis is transmitted by the bite of female phlebotomine
sandflies. The sandflies inject the infective stage, promastigotes,
during blood meals  .
Promastigotes that reach the puncture wound are phagocytized by
macrophages .
Promastigotes that reach the puncture wound are phagocytized by
macrophages  and transform
into amastigotes and transform
into amastigotes  .
Amastigotes multiply in infected cells and affect different tissues,
depending in part on the Leishmania species .
Amastigotes multiply in infected cells and affect different tissues,
depending in part on the Leishmania species
 .
This originates the clinical manifestations of leishmaniasis.
Sandflies become infected during blood meals on an infected host when
they ingest macrophages infected with amastigotes ( .
This originates the clinical manifestations of leishmaniasis.
Sandflies become infected during blood meals on an infected host when
they ingest macrophages infected with amastigotes ( , ,
 ). In the sandfly's
midgut, the parasites differentiate into promastigotes ). In the sandfly's
midgut, the parasites differentiate into promastigotes
 ,
which multiply and migrate to the proboscis ,
which multiply and migrate to the proboscis
 . .
CDC
DPDx Parasite Image Library
|
|
|
Cutaneous
leishmaniasis (Oriental sore, Delhi ulcer, Baghdad boil): In cutaneous
leishmaniasis, the organism (L. tropica) multiplies locally, producing of a
papule, 1-2 weeks (or as long as 1-2 months) after the bite. The papule gradually
grows to form a relatively painless ulcer. The center of the ulcer encrusts
while satellite papules develop at the periphery. The ulcer heals in 2-10
months, even if untreated but leaves a disfiguring scar (figure 12). The disease may
disseminate in the case of depressed immune function.
Mucocutaneous
leishmaniasis (espundia, Uta, chiclero): The initial symptoms of
mucocutaneous leishmaniasis are the same as those of cutaneous leishmaniasis,
except that in this disease the organism can metastasize and the lesions
spread to mucoid (oral, pharyngeal and nasal) tissues and lead to their
destruction and hence sever deformity (figure 12E). The organisms responsible are
L. braziliensis, L. mexicana and L. peruviana.
Pathology
Pathogenesis of leishmaniasis is due to an immune reaction to the organism,
particularly cell mediated immunity. Laboratory examination reveals a marked
leukopenia with relative monocytosis and lymphocytosis, anemia and
thrombocytopenia. IgM and IgG levels are extremely elevated due to both specific
antibodies and polyclonal activation.
Diagnosis
Diagnosis
is based on a history of exposure to sandfies, symptoms and isolation of the
organisms from the lesion aspirate or biopsy, by direct examination or culture.
A skin test (delayed hypersensitivity: Montenegro test) and detection of anti-leishmanial
antibodies by immuno-fluorescence are indicative of exposure.
Treatment and
Control
Sodium stibogluconate (Pentostam) is the drug of choice. Pentamidine isethionate
is used as an alternative. Control measures involve vector control and
avoidance. Immunization has not so far been effective but a
new vaccine
is under investigation.
|
|
Figure
11F |
 Figure 12A
Figure 12A
Skin ulcer due to leishmaniasis, hand of Central American
adult.
CDC/Dr. D.S. Martin
 Figure
12C Figure
12C
Scar on skin of upper leg representing healed lesion of
leishmaniasis
CDC
 Figure 12D
Figure 12D
Non-healing
cutaneous leishmaniasis lesion on ear lobe
WHO/TDR/El-Hassan
 Figure 12E
Figure 12E
Girl with diffuse muco- cutaneous leishmaniasis of the face
which is responding to treatment
WHO/TDR/El-Hassan
 Figure 12F Figure 12F
Cutaneous leishmaniasis skin lesion. The lesion measured about 1 inch in diameter and was moist with raised borders. There was no drainage;
however, the lesion did appear to be infected.
© Lynne S. Garcia, LSG & Associates and
The
Microbe Library
|

Figure
12B
Crater lesion of leishmaniasis, skin CDC |

Figure 12 G
Malaria generally occurs in areas where environmental conditions allow parasite multiplication in the vector. Thus, malaria is usually restricted to tropical and subtropical areas (see map) and altitudes below 1,500 m. However, this distribution might be affected by climatic changes, especially global warming, and population movements. Both
Plasmodium falciparum and P. malariae are encountered in all shaded areas of the map (with P. falciparum by far the most
prevalent). Plasmodium vivax and P. ovale are traditionally thought to occupy complementary niches, with
P. ovale predominating in Sub-Saharan Africa and P. vivax in the other areas; however these two species are not always
distinguishable on the basis of morphologic characteristics alone; the use of molecular tools will help clarify their exact distribution.

Distribution of malaria, 2014
CDC |
MALARIA
Etiology
Four Plasmodium species are responsible for human malaria These are
P.
falciparum, P. vivax, P. ovale and P. malariae.
Epidemiology
There
were an estimated 207 million global cases of malaria in 2012 and at least
627,000 people died of malaria, mostly (over 90%) young children in
sub-Saharan Africa. This makes malaria the leading cause of mortality in
this region. A decade ago, malaria led to the deaths of more
than one million people per year. This drop in mortality, largely as a result of
mosquito control efforts and the use of insecticides within the home, has
cut malaria cases by 45% and saved the lives of 3.3 million people around
the world.
Malaria has been eradicated in
North America and Europe as a result of mosquito control. Yet
travel-associated cases are malaria are still encountered in these regions.
Each year around 2,000 cases of malaria are reported in the United States
with a high of 1,925 in 2011. These are mainly in immigrants who
travel to endemic areas and do not take proper prophylactic measures. These
malaria infections have led to localized outbreaks in the United States as
local mosquitoes acquire the parasite from infected people. In addition,
malaria can be spread as a result of blood transfusions from infected
donors. Between 1863 and 2011, there were 97 such cases.
In the United Kingdom in 2012
there were 1,400 travel-associated cases and two deaths.
P. falciparum (malignant tertian malaria) and
P. malariae (quartan
malaria) are the most common species of malarial parasite and are found in Asia and Africa.
P. vivax
(benign tertian malaria) predominates in Latin America, India and Pakistan,
whereas, P. ovale (ovale tertian malaria) is almost exclusively found in
Africa (figure 12G).
Places where malaria is
endemic:
-
Much of Africa and
southern Asia
-
Central and South
America
-
Some areas of the
Caribbean (Haiti and Dominican Republic)
-
Middle East
-
Some Pacific Island
Morphology
Malarial
parasite trophozoites are generally ring shaped, 1-2 microns in size, although
other forms (ameboid and band) may also exist. The sexual forms of the parasite
(gametocytes) are much larger and 7-14 microns in size. P. falciparum is the
largest and is banana shaped while others are smaller and round. P. vivax causes
stippling of infected red cells (figure 13-17).
 Plasmodium falciparum: Blood Stage Parasites: Thin Blood Smears
Plasmodium falciparum: Blood Stage Parasites: Thin Blood Smears
Fig. 1: Normal red cell; Figs. 2-18: Trophozoites (among these, Figs. 2-10 correspond to ring-stage
trophozoites); Figs. 19-26: Schizonts (Fig. 26 is a ruptured schizont); Figs. 27, 28: Mature
macrogametocytes (female); Figs. 29, 30: Mature microgametocytes (male)
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971 |
 Plasmodium falciparum: Blood Stage Parasites: Thick Blood Smears
Plasmodium falciparum: Blood Stage Parasites: Thick Blood Smears
Illustrations from: Wilcox A. Manual for the Microscopical Diagnosis of Malaria in Man. U.S. Department of Health, Education and Welfare, Washington, 1960.
CDC
|

Plasmodium malariae: Blood Stage Parasites:
Thin Blood Smears
Fig. 1: Normal red cell; Figs. 2-5: Young trophozoites (rings); Figs. 6-13:
Trophozoites; Figs. 14-22: Schizonts; Fig. 23: Developing gametocyte; Fig. 24: Macrogametocyte
(female); Fig. 25: Microgametocyte (male)
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971 |
 Plasmodium malariae: Blood Stage Parasites: Thick Blood Smears
Plasmodium malariae: Blood Stage Parasites: Thick Blood Smears
Illustrations from: Wilcox A. Manual for the Microscopical Diagnosis of Malaria in Man. U.S. Department of Health, Education and Welfare, Washington, 1960.
CDC |
 Plasmodium ovale: Blood Stage Parasites: Thin Blood Smears
Plasmodium ovale: Blood Stage Parasites: Thin Blood Smears
Fig. 1: Normal red cell; Figs. 2-5: Young trophozoites (Rings); Figs. 6-15:
Trophozoites; Figs. 16-23: Schizonts; Fig. 24:
Macrogametocytes (female); Fig. 25: Microgametocyte (male)
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971 |
 Plasmodium vivax: Blood Stage Parasites: Thin Blood Smears
Plasmodium vivax: Blood Stage Parasites: Thin Blood Smears
Fig. 1: Normal red cell; Figs. 2-6: Young trophozoites (ring stage parasites); Figs. 7-18:
Trophozoites; Figs. 19-27: Schizonts; Figs. 28 and 29: Macrogametocytes (female); Fig. 30: Microgametocyte (male)
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971 |
|
Figure 13
Trophozoites: Blood stages malarial parasites
DPDx Parasite Image Library
|
 Plasmodium falciparum: Gametocytes
Plasmodium falciparum: Gametocytes
Figs. 27, 28: Mature macrogametocytes (female); Fig. 29, 30: Mature microgametocytes (male)
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
 Plasmodium malariae: Gametocytes
Plasmodium malariae: Gametocytes
Fig. 23: Developing gametocyte; Fig. 24: Macrogametocyte (female); Fig. 25: Microgametocyte (male)
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|

Plasmodium falciparum: Gametocytes: An asplenic, 41 y.o. woman, immigrant from Haiti, who returned to the US 2 days ago; high P. falciparum
parasitemia; the presence of such young gametocytes in the
peripheral blood is exceptional (specimen contributed by Florida
SHD) CDC |

Plasmodium falciparum: Gametocytes: A patient from Haiti; mature gametocytes (specimen contributed by Florida
SHD) CDC |

Plasmodium malariae: Gametocytes: Smear from patient:
56 y.o. man who had traveled to Kenya (specimen contributed by Wisconsin
SHD) CDC |

Plasmodium malariae: Gametocytes: Smear from patient: 56 y.o. man who had traveled to Kenya (specimen contributed by Wisconsin
SHD) CDC |
 Plasmodium ovale: Gametocytes
Plasmodium ovale: Gametocytes
Fig. 24: Macrogametocyte (female); Fig. 25: Microgametocyte (male).
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
 Plasmodium vivax: Gametocytes
Plasmodium vivax: Gametocytes
Fig. 28 and 29: Nearly mature and mature macrogametocyte (female); Fig. 30: Microgametocyte (male)
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
A
 B
B
 Plasmodium ovale: Gametocytes
Plasmodium ovale: Gametocytes
Smears from patients: Note the Schüffner's dots in A, and the fimbriation of the erythrocyte in B. The erythrocytes in P. ovale infections are less enlarged than with P. vivax, and are not as deformed.
A, B: Male patient born in Nigeria, who came to the US 5 days ago (specimen contributed by Michigan
SHD) CDC |
 A A
 B B
 C Plasmodium vivax: Gametocytes
C Plasmodium vivax: Gametocytes
Smears from patients:
Note the variability in Schüffner's dots.
A: A pregnant woman who visited India 6 months ago (specimen contributed by New Jersey
SHD)
B,C: 50 y.o. woman 3 months ago from a 1-month visit to India (specimen contributed by Indiana SHD)
CDC |
|
Figure 14 Gametocytes
DPDx Parasite Image Library
|

Plasmodium falciparum: Ring Stage Parasites.
Fig. 1: Normal red cell; Figs. 2-10: Increasingly mature ring stage parasites.
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|

Plasmodium malariae: Ring Stage Parasites
Fig. 1: Normal red cell; Figs. 2-5: Rings
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
 Appliqué form
Appliqué form
 Ring with double chromatin dot
Ring with double chromatin dot
 Older ring stage parasite
Older ring stage parasite
 Doubly infected erythrocyte
Doubly infected erythrocyte
 Multiple infections, 6 rings in 2 erythrocytes
Multiple infections, 6 rings in 2 erythrocytes
An asplenic, 41 y.o. woman, immigrant from Haiti, who returned to the US 2 days ago; high P. falciparum
parasitemia (specimen contributed by Florida SHD). CDC |
 Plasmodium malariae: Ring Stage Parasites
Plasmodium malariae: Ring Stage Parasites
Smears from patients:
56 y.o. man who had traveled to Kenya (specimen contributed by Wisconsin
SHD) CDC |
 Plasmodium ovale: Ring Stage Parasites
Plasmodium ovale: Ring Stage Parasites
Fig. 1: Normal red cell; Figs. 2-5: Ring stage parasites
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
 Plasmodium vivax: Ring Stage Parasites
Plasmodium vivax: Ring Stage Parasites
Fig. 1: Normal red cell; Figs. 2-6: Ring stage parasites (young trophozoites)
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
A B
B
 C
C
 Plasmodium ovale: Ring Stage Parasites Smears from patients:
Plasmodium ovale: Ring Stage Parasites Smears from patients:
Note the relatively large chromatin dots. A, C: 54 y.o. man who returned the previous month from a visit to Kenya and Malawi. P.
ovale, confirmed by PCR (specimen contributed by New Mexico SHD). B: 20
y.o. man who returned 10 months ago from a visit to Mozambique, Zimbabwe and Swaziland; this attack is thus a relapse
(specimen contributed by New York SHD). CDC |


 Plasmodium vivax: Ring Stage Parasites Smears from patients:
Plasmodium vivax: Ring Stage Parasites Smears from patients:
A: Rings in 2 slightly enlarged RBCs; 17 y.o. man with a relapse due to P. vivax
(PCR confirmed), 6 months after returning from a visit to Papua New Guinea (specimen contributed by Virginia
SHD)
B: Double infection with rings, RBC enlarged and deformed, Schüffner's dots beginning to become visible; 69
y.o. woman born in India who was symptomatic on the day of arrival to the US (specimen contributed by Pennsylvania
SHD)
C: Late ring in a RBC with Schüffner's dots; 60 y.o. man who returned 2 months ago from a 3 month trip to Laos and North Korea
(specimen contributed by Hawaii SHD) CDC |
|
Figure 15
Ring stage parasites
DPDx Parasite Image Library
|
 Plasmodium falciparum: Schizonts
Plasmodium falciparum: Schizonts
Figs. 19-25: Increasingly mature schizonts; Fig. 26: Ruptured schizont
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
 Plasmodium malariae: Schizonts. Increasingly mature schizonts
Plasmodium malariae: Schizonts. Increasingly mature schizonts
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
A  B
B
 Plasmodium falciparum: Schizonts. Smears from patients: Schizonts are seen only rarely in P. falciparum malaria. An
asplenic, 41 y.o. woman, immigrant from Haiti, who returned to the US 2 days ago; high P. falciparum parasitemia
Plasmodium falciparum: Schizonts. Smears from patients: Schizonts are seen only rarely in P. falciparum malaria. An
asplenic, 41 y.o. woman, immigrant from Haiti, who returned to the US 2 days ago; high P. falciparum parasitemia
A: Young schizont with 10 nuclei;
B: Mature schizont with 24 nuclei, ready to rupture (“segmenter”)
(specimen
contributed by Florida SHD) CDC
|
A
 B
B
 C
C

D
 Plasmodium malariae: Schizonts.Smears from patients:
Plasmodium malariae: Schizonts.Smears from patients:
The parasites are compact and the infected erythrocytes are not enlarged. In C and D, the merozoites are arranged in a rosette pattern.
A, B, C, D: 56 y.o. man who had traveled to Kenya (specimen contributed by Wisconsin
SHD) CDC |

Plasmodium ovale: Schizonts
Increasingly mature schizonts
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
 Plasmodium vivax: Schizonts
Plasmodium vivax: Schizonts
Figs. 19-27: Increasingly mature schizonts
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
A
 B
B
 Plasmodium ovale: Schizonts
Plasmodium ovale: Schizonts
Smears from patients: A, B: 54 y.o. man who returned the previous month from a visit to Kenya and Malawi. Infection with P.
ovale, confirmed by PCR
Note the fimbriation of the erythrocyte in A.
(specimen contributed by New Mexico
SHD). CDC
|
A
 B
B
 C
C
 D
D
 E
E
 Plasmodium vivax: Schizonts Smears from patients: Note that in these patients, the Schüffner's dots are not conspicuous. (This happens in many of the smears received at
CDC; it is probably related to variability in staining.)
Plasmodium vivax: Schizonts Smears from patients: Note that in these patients, the Schüffner's dots are not conspicuous. (This happens in many of the smears received at
CDC; it is probably related to variability in staining.)
A, C, D, E: A pregnant woman who visited India 6 months ago (specimen contributed by New Jersey
SHD)
B: 17 y.o. man with a relapse due to P. vivax (PCR confirmed) (specimen contributed by Virginia
SHD) CDC |
|
Figure
16 Schizonts
DPDx Parasite Image Library
|
 Plasmodium falciparum: Trophozoites
Plasmodium falciparum: Trophozoites
Figs. 11-18: Increasingly mature trophozoites
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
 Plasmodium malariae: Trophozoites
Plasmodium malariae: Trophozoites
Figs. 6-13: Increasingly mature trophozoites; Fig. 13 is a "band form".
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
A
 B
B  Thin smears from two patients with high parasitemias: A: An
asplenic, 41 y.o. woman, immigrant from Haiti, who returned to the US 2 days ago; high P. falciparum parasitemia (specimen contributed by Florida
SHD) CDC
Thin smears from two patients with high parasitemias: A: An
asplenic, 41 y.o. woman, immigrant from Haiti, who returned to the US 2 days ago; high P. falciparum parasitemia (specimen contributed by Florida
SHD) CDC
B: A patient who acquired malaria by blood transfusion and died with extremely high
parasitemia; PCR confirmed P. falciparum; one of the 2 RBCs contains 3 young
trophozoites, which have begun to accumulate pigment
(specimen contributed by Missouri SHD); CDC |
A
 B
B
 C
C
 Plasmodium malariae: Trophozoites Smears from patients:
Plasmodium malariae: Trophozoites Smears from patients:
The infected erythrocytes are not enlarged (sometime they even appear smaller than non-infected ones). C is a "band form"
trophozoite.
A, B, C: 56 y.o. man who had traveled to Kenya (specimen contributed by Wisconsin
SHD) CDC |
 Plasmodium ovale: Trophozoites
Plasmodium ovale: Trophozoites
Increasingly mature trophozoites. Note the fimbriated red cells (Figs. 8, 13)
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|
 Plasmodium vivax: Trophozoites
Plasmodium vivax: Trophozoites
Figs. 8-18: Increasingly mature trophozoites of P. vivax
CDC Illustrations from: Coatney
GR, Collins WE, Warren M, Contacos PG. The Primate Malarias. U.S. Department of Health, Education and Welfare, Bethesda,
1971
|


 Plasmodium ovale: Trophozoites Smears from patients: Note the lack of ameboidicity in the older trophozoites
(B,C) and the fimbriation of the erythrocyte in C. The erythrocytes in P. ovale infections are less enlarged than with P.
vivax, and are not as deformed. The Schüffner's dots are visible in A, but not B and C.
Plasmodium ovale: Trophozoites Smears from patients: Note the lack of ameboidicity in the older trophozoites
(B,C) and the fimbriation of the erythrocyte in C. The erythrocytes in P. ovale infections are less enlarged than with P.
vivax, and are not as deformed. The Schüffner's dots are visible in A, but not B and C.
A: 20 y.o. man who returned 10 months ago from a visit to Mozambique, Zimbabwe and Swaziland (specimen contributed by New York
SHD). CDC
B, C: 23 y.o. man who arrived to the US 5 months ago after having been in Liberia and Ivory Coast
(specimen contributed by Kentucky SHD) CDC |
A
 B
B
 C
C

D
 E
E
 Plasmodium vivax: Trophozoites
Plasmodium vivax: Trophozoites
Smears from patients: Increasingly mature trophozoites. The RBCs are enlarged and deformed, the parasites are
ameboid, and the Schüffner's dots vary in intensity.
A, B: 26 y.o. woman who spent 2 weeks in Papua New Guinea 5 months ago (specimen contributed by Pennsylvania
SHD) CDC
C, E: 60 y.o. man who returned 2 months ago from a 3-month visit to Laos and North Korea (specimen contributed by Hawaii
SHD)
D: 28 y.o. woman who returned 3 months ago from a 2 weeks visit to Kenya (specimen contributed by Texas
SHD) CDC |
|
Figure 17
Trophozoites
DPDx Parasite Image Library
|
|
 Figure
18 Figure
18
The malaria parasite life cycle
involves two hosts. During a blood meal, a malaria-infected female
Anopheles mosquito inoculates sporozoites into the human host
 .
Sporozoites infect liver cells .
Sporozoites infect liver cells
 and mature into schizonts
and mature into schizonts  ,
which rupture and release merozoites ,
which rupture and release merozoites
 .
(Of note, in P. vivax and P. ovale a dormant stage [hypnozoites]
can persist in the liver and cause relapses by invading the bloodstream
weeks, or even years later.) After this initial replication in the
liver (exo-erythrocytic schizogony .
(Of note, in P. vivax and P. ovale a dormant stage [hypnozoites]
can persist in the liver and cause relapses by invading the bloodstream
weeks, or even years later.) After this initial replication in the
liver (exo-erythrocytic schizogony
 ),
the parasites undergo asexual multiplication in the erythrocytes (erythrocytic
schizogony ),
the parasites undergo asexual multiplication in the erythrocytes (erythrocytic
schizogony  ). Merozoites
infect red blood cells ). Merozoites
infect red blood cells  .
The ring stage trophozoites mature into schizonts, which rupture
releasing merozoites .
The ring stage trophozoites mature into schizonts, which rupture
releasing merozoites  .
Some parasites differentiate into sexual erythrocytic stages
(gametocytes) .
Some parasites differentiate into sexual erythrocytic stages
(gametocytes)  . Blood
stage parasites are responsible for the clinical manifestations of the
disease. . Blood
stage parasites are responsible for the clinical manifestations of the
disease.
The gametocytes, male (microgametocytes) and female (macrogametocytes),
are ingested by an Anopheles mosquito during a blood meal
 .
The parasites’ multiplication in the mosquito is known as the
sporogonic cycle .
The parasites’ multiplication in the mosquito is known as the
sporogonic cycle  .
While in the mosquito's stomach, the microgametes penetrate the
macrogametes generating zygotes .
While in the mosquito's stomach, the microgametes penetrate the
macrogametes generating zygotes
 .
The zygotes in turn become motile and elongated (ookinetes) .
The zygotes in turn become motile and elongated (ookinetes)
 which invade the midgut wall of the mosquito where they develop into
oocysts
which invade the midgut wall of the mosquito where they develop into
oocysts  . The oocysts
grow, rupture, and release sporozoites . The oocysts
grow, rupture, and release sporozoites
 ,
which make their way to the mosquito's salivary glands.
Inoculation of the sporozoites into a new human host perpetuates the
malaria life cycle ,
which make their way to the mosquito's salivary glands.
Inoculation of the sporozoites into a new human host perpetuates the
malaria life cycle  .
CDC
DPDx Parasite Image Library .
CDC
DPDx Parasite Image Library
|
Life
cycle
Malarial
parasites are transmitted by the infected female anopheline mosquito which injects
sporozoites present in the saliva of the insect (Figure 18). Sporozoites infect
the liver parenchymal cells where they may remain dormant (hypnozoites) or
undergo stages of schizogony to produce schizonts and merogony to produce
merozoites (meronts). When parenchymal cells rupture, thousands of meronts are
released into blood and infect the red cells. P. ovale and P. vivax infect
immature red blood cells whereas P. malariae infects mature red cells. P. falciparum
infects
both. In red cells, the parasites mature into trophozoites. These trophozoites undergo
schizogony and merogony in red cells which ultimately burst and release daughter
merozoites. Some of the merozoites transform into male and female gametocytes
(figure 19) while others enter red cells to continue the erythrocytic cycle. The gametocytes
are ingested by the female mosquito, the female gametocyte transforms into
ookinete, is fertilized, and forms an oocyst (figure 20) in the gut. The oocyte produces
sporozoites (sporogony) (figure 20) which migrate to the salivary gland and are ready to
infect another host. The liver (extraerythrocytic) cycle takes 5-15
days whereas the erythrocytic cycle takes 48 hours or 72 hours (P. malariae).
Malaria can be transmitted by transfusion and transplacentally.
 Stage II (central) and stage III (bottom right) immature gametocytes
(blood film, wet mount, x1000 magnification under oil immersion) Image courtesy of Dr Andrew
Taylor-Robinson, University of Leeds, UK © Dr Andrew
Taylor-Robinson
Stage II (central) and stage III (bottom right) immature gametocytes
(blood film, wet mount, x1000 magnification under oil immersion) Image courtesy of Dr Andrew
Taylor-Robinson, University of Leeds, UK © Dr Andrew
Taylor-Robinson |
 Stage IV immature gametocyte, located centrally (blood film, wet mount,
x400 magnification) Image courtesy of Dr Andrew
Taylor-Robinson, University of Leeds, UK © Dr Andrew
Taylor-Robinson
Stage IV immature gametocyte, located centrally (blood film, wet mount,
x400 magnification) Image courtesy of Dr Andrew
Taylor-Robinson, University of Leeds, UK © Dr Andrew
Taylor-Robinson |
 Stage V mature gametocyte, showing characteristic sausage-shaped
morphology, located centrally (blood film, wet mount, x1000
magnification under oil immersion) Image courtesy of Dr Andrew
Taylor-Robinson, University of Leeds, UK © Dr Andrew
Taylor-Robinson
Stage V mature gametocyte, showing characteristic sausage-shaped
morphology, located centrally (blood film, wet mount, x1000
magnification under oil immersion) Image courtesy of Dr Andrew
Taylor-Robinson, University of Leeds, UK © Dr Andrew
Taylor-Robinson |
 Male (micro)gametocyte exflagellation - extrusion of motile,
flagella-like microgametes with vigorous movement (blood film, wet
mount, x1000 magnification under oil immersion) (an unusually clear
picture of this metabolically dynamic and visually striking event)
Male (micro)gametocyte exflagellation - extrusion of motile,
flagella-like microgametes with vigorous movement (blood film, wet
mount, x1000 magnification under oil immersion) (an unusually clear
picture of this metabolically dynamic and visually striking event)
Image courtesy of Dr Andrew
Taylor-Robinson, University of Leeds, UK © Dr Andrew
Taylor-Robinson |
|
Figure 19 Sexual
stages of the malaria parasite Plasmodium falciparum |
 Two oocysts, dissected from the outer wall of the Anopheles stephensi
midgut, 10 days post infection of the mosquito (wet mount, x400
magnification)
Two oocysts, dissected from the outer wall of the Anopheles stephensi
midgut, 10 days post infection of the mosquito (wet mount, x400
magnification)
Image courtesy of Dr Andrew
Taylor-Robinson, University of Leeds, UK © Dr Andrew
Taylor-Robinson |
 Single oocyst, dissected from the outer wall of the Anopheles stephensi
midgut, 10 days post infection of the mosquito (wet mount, x400
magnification)
Single oocyst, dissected from the outer wall of the Anopheles stephensi
midgut, 10 days post infection of the mosquito (wet mount, x400
magnification)
Image courtesy of Dr Andrew
Taylor-Robinson, University of Leeds, UK © Dr Andrew
Taylor-Robinson |
 Single oocyst, dissected from the outer wall of the Anopheles stephensi
midgut, 10 days post infection of the mosquito (wet mount, x1000
magnification under oil immersion)
Single oocyst, dissected from the outer wall of the Anopheles stephensi
midgut, 10 days post infection of the mosquito (wet mount, x1000
magnification under oil immersion)
Image courtesy of Dr Andrew
Taylor-Robinson, University of Leeds, UK © Dr Andrew
Taylor-Robinson |
 Isolated bow-shaped sporozoite, dissected from the salivary glands of
Anopheles stephensi, 17 days post infection of the mosquito (wet mount,
x1000 magnification under oil immersion)
Isolated bow-shaped sporozoite, dissected from the salivary glands of
Anopheles stephensi, 17 days post infection of the mosquito (wet mount,
x1000 magnification under oil immersion)
Image
courtesy of Dr Andrew Taylor-Robinson, University of Leeds, UK ©
Dr Andrew Taylor-Robinson |
|
Figure 20
Developmental stages of Plasmodium falciparum in the Anopheles mosquito
vector |
|
|
FLASH MOVIE
Life
cycle of Plasmodium
WHO/Welcome
Trust
|

Figure 20B
Positive malaria IFA showing a fluorescent schizont
CDC DPDx Parasite Image Library |
Symptoms
The
symptomatology of malaria depends on the parasitemia, the presence of the organism
in different organs and the parasite burden. The incubation period varies
generally between 10 to 30 days. As the parasite load becomes significant, the
patient develops headache, lassitude, vague pains in the bones and joints,
chilly sensations and fever. As the disease progresses, the chills and fever
become more prominent. The chill and fever follow a cyclic pattern (paroxysm)
with the symptomatic period lasting 8 to 12 hours. In between the symptomatic
periods, there is a period of relative normalcy, the duration of which depends
upon the species of the infecting parasite. This interval is about 34 to 36 hours
in the case of P. vivax and P. ovale (tertian malaria), and 58 to 60 hours in the
case of P. malariae (quartan malaria). Classical tertian paroxysm is rarely seen
in P. falciparum and persistent spiking or a daily paroxysm is more usual.
The malarial
paroxysm is most dramatic and frightening. It begins with a chilly sensation
that progresses to teeth chattering, overtly shaking chill and peripheral
vasoconstriction resulting in cyanotic lips and nails (cold stage). This lasts
for about an hour. At the end of this period, the body temperature begins to
climb and reaches 103 to 106 degrees F (39 to 41degrees C). Fever is associated with severe
headache, nausea (vomiting) and convulsions. The patient experiences euphoria,
and profuse perspiration and the temperature begins to drop. Within a few hours
the patient feels exhausted but symptom-less and remains asymptomatic until the
next paroxysm. Each paroxysm is due to the rupture of infected erythrocytes and
release of parasites.
Without treatment, all
species of human malaria may ultimately result in spontaneous cure except with
P. falciparum which becomes more severe progressively and results in death. This
organism causes sequestration of capillary vasculature in the brain,
gastrointestinal and renal tissues. Chronic malaria results in splenomegaly,
hepatomegaly and nephritic syndromes. Nevertheless, malaria is treatable if
caught early enough.
Patients infected with P.
ovale and P. vivax may experience relapses over a period of
months or years. During remission periods, the parasite (hypnozoite) lies
dormant in the liver.
Summary of symptoms
In the first 8 to 12 hours
of overt disease, the following symptoms are experienced:
-
Chills: Feeling cold and
shivering
-
Fever: Feeling hot with
headache and nausea. There may be seizures in children
-
Sweats: The patient's
fever subsides and temperature falls. The patient feels weary with
general malaise and aches
This is then followed by a
cyclic pattern of similar symptoms, the intervals between cycles
depending on the type of infection.
This may be followed by a
more severe disease in which organ collapse occurs and many erythrocytes
contain parasites:
-
Infection of the brain
leading to seizures, coma and behavioral changes
-
Anemia resulting from
erythrocyte lysis
-
Hemoglobin in the urine
(hemoglobinurea)
-
Lung inflammation
resulting in respiratory distress
-
Cardiovascular collapse
from low blood pressure
-
Kidney failure
-
Acidosis and
hypoglycemia
Pathology and
immunology
Symptoms of malaria are due to the release of massive number of
merozoites into the circulation. Infection results in the production of antibodies
which are effective in containing the parasite load. These antibodies are
against merozoites and schizonts. The infection also results in the activation
of the reticuloendothelial system (phagocytes). The activated macrophages help
in the destruction of infected (modified) erythrocytes and antibody-coated
merozoites. Cell mediated immunity also may develop and help in the elimination
of infected erythrocytes. Malarial infection is associated with
immunosuppression.
Diagnosis
Diagnosis
is based on symptoms and detection of parasite in Giemsa stained blood smears.
There are also antibody tests (Figure 20B). Other laboratory findings may
include: thrombocytopenia, high bilirubin, high aminotransferases.
Treatment and
Control
Treatment is effective with various quinine derivatives (quinine sulphate,
chloroquine, meflaquine and primaquine, etc.). Drug resistance, particularly in
P. falciparum and to some extent in P. vivax is a major problem. Control
measures are eradication of infected anopheline mosquitos. Vaccines are being
developed and tried but none is available yet for routine use.
An infection (particularly with
P. falciparum ) may be treated with a variety of drugs among which
are:
-
Chloroquine
This binds to heme, released from hemoglobin in the parasitized
erythrocyte, to form a complex that is toxic to and lyzes the
erythrocyte.
-
Atovaquone-proguanil (Malarone)
Atovaquone is a mitochondrial electron transfer blocker. Proguanil is a
prodrug that is metabolized to cycloguanil that blocks dihydrofolate
reductase and also enzymes involved in DNA production.
-
Mefloquine (Lariam)
This drug is widely used as a prophylactic, especially where chloroquine-resistant
plasmodia occur. It can have serious side effects in some people. It has
been proposed to interact with an erythrocyte membrane protein called
stomatin and is then transferred to the parasite's membrane.
-
Artemether-lumefrantine (Coartem).
Artemether is a semi-synthetic derivative of artemisinin and is
metabolized to dihydroartemisinin which may act via an endoperoxide
moiety. The mechanism of action of lumefrantine, which is a synthetic
compound, is unknown. This drug combination is used to treat acute
uncomplicated P. falciparum malaria.
-
Quinine
This drug has been around since the 17th century and was the first
effective anti-malarial in western medicine. It comes from the
bark of the cinchona tree. The mechanism of action is not known but is
proposed to be similar to choroquine. Quinidine is a stero isomer of
quinine
-
Doxycycline (combined with
quinine)
In addition to be an anti-bacterial agent, doxycyline is anti-protozoal
and is used in malaria prophylaxis. It may alter the division of a
plastid found in protozoan cells called the apicoplast.
-
Clindamycin (combined
with quinine)
This is also an anti-bacterial but has action against some protozoans.
It acts on the ribosome.
-
Artesunate
This is not license for use in the United States. Like artemether, this
is a semi-synthetic derivative of artemisinin.
Prophylaxis treatment often
includes: Atovaquone-proguanil or Mefloquine as chloroqine-resistance is
becoming increasingly common.
|
|
|
|
CASE REPORT
One Traveler’s Ordeal with Severe Malaria: A
Cautionary Tale
CDC |

Figure 21A
Babesia microti infection, Giemsa-stained thin smear. The organisms resemble
Plasmodium falciparum; however Babesia parasites present several
distinguishing features: they vary more in shape and in size; and they do not produce pigment. A 67 year old woman, status
post-splenectomy, infection probably acquired in Long island (New York)
CDC |
BABESIOSIS
Babesiosis, like malaria, is an infection
of erythrocytes. It is spread by ticks.
Etiology
Babesia
microti is the most important member of the genus that infects man, although
a few cases of infection by Babesia sp. have been detected.
Epidemiology
In the United States, infections are usually seen in the northeast
and the upper mid-west (figure 21E) during the summer months (figure
21F) when ticks are more likely to come in contact with
humans. In 2012, there were 911 reported cases of babeosis (figure
21G). Patients had a median age of 62 (figure 21H) and two thirds
were male, probably reflecting the fact that men are more likely to
come in contact with Ixodid ticks.
Morphology
The
trophozoite is very similar to the ring form of the Plasmodium species (figure
21A and B).
|
|
 Figure 21B
Figure 21B
Infection with Babesia. Giemsa-stained thin smears. Note the tetrad (left side of the image), a dividing form pathognomonic for
Babesia. A 6 year old girl, status post splenectomy for hereditary
spherocytosis, infection acquired in the US.
CDC
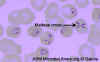 Figure 21C
Figure 21C
Thin blood film of B.
microti ring forms with a typical Maltese Cross (four rings in cross
formation).
© MicrobeLibrary and Lynne Garcia,
LSG & Associates |
Life cycle
The
organism (sporozoite) is transmitted by a tick (Ixodes scapularis) and enters the red cell where it
undergoes mitosis and the organisms (merozoite) are released to infect other red
cells. Ticks acquire the organism during feeding on an infected
individual. In the tick, the organism divides sexually in the gut and migrates
into the salivary gland (figure 21D).
Babesiosis has also be spread by blood transfusion and from other to
fetus.Symptoms
Infections are often asymptomatic and in others there are flu-like
symptoms:
-
fever
-
malaise
-
chills sweats
-
general aches and
pains
However, the destruction
of erythrocytes can lead to:
-
hemolytic anemia
-
jaundice
-
hepatomegaly
These occur usually
1 to 2 weeks after infection. Although usually not severe, babeosis can be
life-threatening as a result of additional complications including
thrombocytopenia, low blood pressure, disseminated intravascular
coagulation (consumptive coagulopathy) leading to thromboses, and
organ collapse. This can be fatal, especially in immunosuppressed
patients, the elderly and those that have undergone
splenectomy.
Diagnosis
Diagnosis
is based on symptoms, patient history and detection of intraerythrocytic
parasite in the blood (figure 21B,D) or transfer of blood in normal hamsters which can be
heavily parasitized.
Treatment and
Control
Drugs of choice are clindamycin combined with quinine or
atovaquone combined with azithromycin.
The patient may recover
spontaneously. One should avoid tick exposure and, if bitten, remove the tick from
the skin
immediately.
|
| |
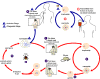 Figure 21D
Figure 21D
The
Babesia microti life cycle involves two hosts, which includes a
rodent, primarily the white-footed mouse, Peromyscus leucopus.
During a blood meal, a Babesia-infected tick introduces
sporozoites into the mouse host
 .
Sporozoites enter erythrocytes and undergo asexual reproduction
(budding) .
Sporozoites enter erythrocytes and undergo asexual reproduction
(budding)  . In the
blood, some parasites differentiate into male and female gametes
although these cannot be distinguished at the light microscope level . In the
blood, some parasites differentiate into male and female gametes
although these cannot be distinguished at the light microscope level
 .
The definitive host is a tick, in this case the deer tick, Ixodes
dammini (I. scapularis). Once ingested by an
appropriate tick .
The definitive host is a tick, in this case the deer tick, Ixodes
dammini (I. scapularis). Once ingested by an
appropriate tick  , gametes
unite and undergo a sporogonic cycle resulting in sporozoites , gametes
unite and undergo a sporogonic cycle resulting in sporozoites
 .
Transovarial transmission (also known as vertical, or hereditary,
transmission) has been documented for “large” Babesia spp.
but not for the “small” babesiae, such as B. microti .
Transovarial transmission (also known as vertical, or hereditary,
transmission) has been documented for “large” Babesia spp.
but not for the “small” babesiae, such as B. microti
 .
Humans enter the cycle when bitten by infected ticks. During a
blood meal, a Babesia-infected tick introduces sporozoites into
the human host .
Humans enter the cycle when bitten by infected ticks. During a
blood meal, a Babesia-infected tick introduces sporozoites into
the human host  .
Sporozoites enter erythrocytes .
Sporozoites enter erythrocytes
 and undergo asexual replication (budding)
and undergo asexual replication (budding)
 .
Multiplication of the blood stage parasites is responsible for the
clinical manifestations of the disease. Humans are, for all
practical purposes, dead-end hosts and there is probably little, if any,
subsequent transmission that occurs from ticks feeding on infected
persons. However, human to human transmission is well recognized
to occur through blood transfusions .
Multiplication of the blood stage parasites is responsible for the
clinical manifestations of the disease. Humans are, for all
practical purposes, dead-end hosts and there is probably little, if any,
subsequent transmission that occurs from ticks feeding on infected
persons. However, human to human transmission is well recognized
to occur through blood transfusions
 .
Note:
Deer are the hosts upon which the adult ticks feed and are indirectly
part of the Babesia cycle as they influence the tick population.
When deer populations increase, the tick population also increases, thus
heightening the potential for transmission. .
Note:
Deer are the hosts upon which the adult ticks feed and are indirectly
part of the Babesia cycle as they influence the tick population.
When deer populations increase, the tick population also increases, thus
heightening the potential for transmission.
CDC
DPDx Parasite Image Library
 Figure 21E
Figure 21E
Number of reported cases of babesiosis, by county of residence — 27
states, 2013
CDC
 Figure 21F
Figure 21F
Number of reported cases of babesiosis, by month of symptom onset — 2013
CDC
 Figure 21G
Figure 21G
Number of reported cases of babesiosis, by year
CDC
 Figure 21H
Figure 21H
Number of reported cases of babesiosis, by age group — 2013
CDC
|

Figure
22 Members
of the cat family (Felidae) are the only known definitive hosts for the
sexual stages of T. gondii and thus are the main reservoirs of
infection. Cats become infected with T. gondii by
carnivorism (1).
After tissue cysts or oocysts are ingested by the cat, viable organisms
are released and invade epithelial cells of the small intestine where
they undergo an asexual followed by a sexual cycle and then form oocysts,
which are then excreted. The unsporulated oocyst takes 1 to 5 days
after excretion to sporulate (become infective). Although cats
shed oocysts for only 1 to 2 weeks, large numbers may be shed.
Oocysts can survive in the environment for several months and are
remarkably resistant to disinfectants, freezing, and drying, but are
killed by heating to 70°C for 10 minutes.
Human infection may be acquired in several ways: A) ingestion of
undercooked infected meat containing Toxoplasma cysts (2);
B) ingestion of the oocyst from fecally contaminated hands or food (3);
C) organ transplantation or blood transfusion; D) transplacental
transmission; E) accidental inoculation of tachyzoites. The
parasites form tissue cysts, most commonly in skeletal muscle,
myocardium, and brain; these cysts may remain throughout the life of the
host.
CDC DPDx Parasite Image Library |
TOXOPLASMOSIS
Etiology
Toxoplasma
gondii is the organism responsible for toxoplasmosis
Epidemiology
Toxoplasma has worldwide distribution and 20%-75% of the population is
seropositive without any symptomatic episode.
In the United States, 22.5% of the population is seropositive. However, the infection poses a
serious threat in immunosuppressed individuals and pregnant females.
The most common routes for human
infection are:
Toxoplasma may also be spread
congenitally (from a mother with no symptoms) and rarely via blood
transfusions and organ transplants.
Morphology
The
intracellular parasites (tachyzoite) are 3x6 microns, pear-shaped organisms that
are enclosed in a parasite membrane to form a cyst measuring 10-100 microns in
size. Cysts in cat feces (oocysts) are 10-13 microns in diameter (figure 22).
Life cycle
The
natural life cycle of T. gondii occurs in cats and small rodents, although the
parasite can grow in the organs (brain, eye, skeletal muscle, etc.) of any
mammal or birds (Figure 22). Cats gets infected by ingestion of cysts in flesh.
Decystation occurs in the small intestine, and the organisms penetrate the
submucosal epithelial cells where they undergo several generations of mitosis,
finally resulting in the development of micro- (male) and macro- (female)
gametocytes. Fertilized macro-gametocytes develop into oocysts that are
discharged into the gut lumen and excreted. Oocysts sporulate in the warm
environment and are infectious to a variety of animals including rodents and
man. Sporozoites released from the oocyst in the small intestine penetrate the
intestinal mucosa and find their way into macrophages where they divide very
rapidly (hence the name tachyzoites) (figure 23) and form a cyst which may occupy the whole
cell. The infected cells ultimately burst and release the tachyzoites to enter
other cells, including muscle and nerve cells, where they are protected from the
host immune system and multiply slowly (bradyzoites). These cysts are infectious
to carnivores (including man). Unless man is eaten by a cat, it is a dead-end
host.
Symptoms
Although
Toxoplasma infection is common, it rarely produces symptoms in normal
individuals and when symptoms do occur, they are flu-like and sometimes
associated with lymphadenopathy. Serious consequences are limited to pregnant women and immunodeficient hosts.
Congenital infections
These occur in about 1 to 5 per 1000
pregnancies of which 5 to 10% result in miscarriage and 8 to 10% result in serious brain
and eye damage to the fetus. 10 to 13% of the babies will have visual handicaps.
Although 58 to 70% of infected women will give birth to a normal offspring, a small proportion
of babies will develop active retino-chorditis or mental retardation in
childhood or young adulthood. Eye lesions are often not identified at birth but
are found in 20 to 80% of infected patients by adulthood. In the United
States fewer than 2% of patients develop eye lesions.
Immunocompromized
patients
In immunocompromized individuals, infection results in generalized parasitemia
involvement of brain, liver lung and other organs, and often death.
Immunology
Both
humoral and cell mediated immune responses are stimulated in normal individuals.
Cell-mediated immunity is protective and humoral response is of diagnostic value.
Diagnosis
Suspected
toxoplasmosis can be confirmed by isolation of the organism from tonsil or lymph
gland biopsy and by serologic testing.
Treatment
Acute
infections benefit from pyrimethamine or sulphadiazine. Spiramycin is a
successful alternative. Pregnant women are advised to avoid cat litter and to handle
uncooked and undercooked meat carefully.
|
|
Figure
23
|
 Figure 23A Figure 23A
Toxoplasma gondii in the bronchoalveolar lavage (BAL) material from an HIV infected patient. Numerous
trophozoites (tachyzoites) can be seen, which are typically crescent shaped with a
prominent, centrally placed nucleus. Most of the tachyzoites are free, some are still associated with bronchopulmonary cells.
CDC  Figure 23B Figure 23B
Toxoplasma gondii in tissue from a cat.
CDC
 Figure 23C
Figure 23C
Toxoplasma gondii in mouse
ascitic fluid. Smear
CDC
|
| |
PNEUMOCYSTIS
PNEUMONIA
Pneumocystis
jiroveci (formerly known as Pneumocystis carinii)
Pneumocystis
jiroveci was formerly thought to be a protozoan but is now known to be a
fungus. It is included here because pneumocystis pneumonia is often
described as an opportunistic parasitic disease.
Pneumocystis pneumonia
is an infection
of immunosuppressed individuals and is particularly seen in AIDS patients. In
the United States, about 10% of AIDS patients and about 1% of solid
organ transplant recipients are infected.
The organism is pleomorphic, exhibiting, at various stages of its life cycle: 1-2 micron sporozoites, 4-5
micron trophozoites and 6-8 micron
cysts. It spreads from person to person in cough droplets. Infection in
immunosuppressed individuals results in interstitial pneumonia characterized by
thickened alveolar septum infiltrated with lymphocytes and plasma cells.
Pneumonia is associated with fever,
tachypnea, hypoxia,
cyanosis and asphyxia.
Diagnosis is based on isolation of organisms from affected lungs.
Trimethoprim-sulphamethoxazole is the treatment of choice (figure 24).
The mortality rate for P. jiroveci infections is 5 to 40%
when treated and near 100% when untreated.
|
|
|
|
|
 Figure 24A
Figure 24A
Pneumocystis jiroveci trophozoites in broncho-alveolar lavage (BAL) material. Giemsa stain. The trophozoite are small (size: 1-5 µm), and only their nuclei, stained purple, are visible (arrows). AIDS
patient seen in Atlanta, Georgia
CDC

 Figure
24 B and C Figure
24 B and C
Pneumocystis jiroveci cysts
B. 3 cysts in bronchoalveolar material, Giemsa stain; the rounded cysts (size 4-7 µm) contain 6-8 intracystic bodies, whose nuclei are stained by
Giemsa; the walls of the cysts
are not stained; note the presence of several smaller, isolated
trophozoites.
C. cysts in lung tissue, silver stain; the walls of the cysts are stained black; the intracystic bodies are not visible with this stain; baby
who died with pneumonia in California. CDC
 Figure 24D Figure 24D
This
is a generalized life cycle proposed by John J. Ruffolo, Ph.D. (Cushion,
MT, 1988) for the various species of Pneumocystis. These
fungi are found in the lungs of mammals where they reside without
causing overt infection until the host's immune system becomes
debilitated. Then, an oftentimes lethal pneumonia can result.
Asexual phase: trophic forms  replicate by mitosis
replicate by mitosis  to to
 .
Sexual phase: haploid trophic forms conjugate .
Sexual phase: haploid trophic forms conjugate
 and produce a zygote or sporocyte (early cyst)
and produce a zygote or sporocyte (early cyst)
 .
The zygote undergoes meiosis and subsequent mitosis to produce
eight haploid nuclei (late phase cyst) .
The zygote undergoes meiosis and subsequent mitosis to produce
eight haploid nuclei (late phase cyst)
 .
Spores exhibit different shapes (such as, spherical and elongated
forms). It is postulated that elongation of the spores
precedes release from the spore case. It is believed that the
release occurs through a rent in the cell wall. After release, the
empty spore case usually collapses, but retains some residual cytoplasm .
Spores exhibit different shapes (such as, spherical and elongated
forms). It is postulated that elongation of the spores
precedes release from the spore case. It is believed that the
release occurs through a rent in the cell wall. After release, the
empty spore case usually collapses, but retains some residual cytoplasm
 .
A trophic stage, where the organisms probably multiply by binary fission
is also recognized to exist. The organism causes disease in
immunosuppressed individuals. .
A trophic stage, where the organisms probably multiply by binary fission
is also recognized to exist. The organism causes disease in
immunosuppressed individuals.
Pneumocystis
stages were reproduced from a drawing by Dr. John J. Ruffolo, South
Dakota State University, USA. Reproduced by permission of Arnold
and Dr. Ruffolo. Thanks to Dr. Melanie T. Cushion for her comments on
the life cycle text. References:
Ruffolo JJ. Pneumocystis carinii Cell Structure. In:
Walzer, PD, editor. Pneumocystis carinii Pneumonia. 2nd
ed. Marcel Dekker; 1994. p. 25-43.
Cushion MT, Ruffolo JJ, Walzer PD. Analysis of the developmental
stages of Pneumocystis carinii in vitro. Lab Invest
1988;58:324-331.
CDC DPDx Parasite Image Library |
| |
| |
FACULTATIVE
PARASITIC PROTOZOA
These are free-living
amebae that occasionally cause serious human disease. They are of particular
significance in immunocompromised hosts.
Naegleria
fowleri
This organism causes a rare
disease. It is a flagellate that may inhabit warm waters (spas, warm springs, heated
swimming pools, etc.) and gain access via the nasal passage to the brain and
cause primary amebic
meningoencephalitis which is almost always fatal (figure 25). Only three
people out of 132 have survived primary amebic meningoencephalitis in the
last 50 years.
Naegleria fowleri is
sometimes call "the brain eating ameba". Although Naegleria can be
found in contaminated tap water, human infection does not result from
drinking the water.
Epidemiology
In the United States,
infections are rare with only 34 cases between 2004 and 2013. These
resulted from
-
Contaminated
recreational water (3o cases)
-
Nasal irrigation with
contaminated tap water (3 cases)
-
Contaminated tap water
on a backyard slide (1 case)
Symptoms
One to seven days after nasal exposure, the patient suffers:
-
Sever headache
-
Nausea/vomiting
-
Fever
As the meningoencephalitis
develops, patients then experience:
Treatment
There is an investigational drug, miltefosine, that may show promise. In
2013, two children survived an infection. One started treatment 36 hours
after onset of treatment. She was treated with therapeutic hypothermia
and miltefosine. She made a complete recovery. Another child did not
receive hypothermia and was treated later after the onset of symptoms.
He did receive miltefosine. He suffered permanent brain damage.
|
|
|
 Figure 25 A
Figure 25 A
Naegleria fowleri trophozoites, cultured from cerebrospinal fluid. These cells have characteristically large nuclei, with a large, dark staining
karyosome. The amebae are very active and extend and retract pseudopods. Trichrome stain. From a patient who died from primary amebic meningoencephalitis in Virginia.
CDC
 Figure 25B
Figure 25B
Naegleria fowleri trophozoite in spinal fluid. Trichrome stain. Note the typically large karyosome and the monopodial locomotion. Image contributed by Texas
SHD.
CDC
 Figure 25C Figure 25C
Histopathology of amebic meningoencephalitis due to
Naegleria
fowleri. Direct fluorescent antibody stain.
CDC/Dr. Govinda S. Visvesvara gsv1@cdc.gov
 Figure 25D
Figure 25D
Histopathology of Naegleria infection of brain.
CDC
 Figure
25E
Figure
25E
Free-living
amebae belonging to the genera Acanthamoeba, Balamuthia,
and Naegleria are important causes of disease in humans and
animals. Naegleria fowleri produces an acute, and usually
lethal, central nervous system (CNS) disease called primary amebic
meingoencephalitis (PAM). N. fowleri has three stages,
cysts  , trophozoites , trophozoites
 ,
and flagellated forms ,
and flagellated forms  , in
its life cycle. The trophozoites replicate by promitosis (nuclear
membrane remains intact) , in
its life cycle. The trophozoites replicate by promitosis (nuclear
membrane remains intact)  .
Naegleria fowleri is found in fresh water, soil, thermal
discharges of power plants, heated swimming pools, hydrotherapy and
medicinal pools, aquariums, and sewage. Trophozoites can turn into
temporary flagellated forms which usually revert back to the trophozoite
stage. Trophozoites infect humans or animals by entering the
olfactory neuroepithelium .
Naegleria fowleri is found in fresh water, soil, thermal
discharges of power plants, heated swimming pools, hydrotherapy and
medicinal pools, aquariums, and sewage. Trophozoites can turn into
temporary flagellated forms which usually revert back to the trophozoite
stage. Trophozoites infect humans or animals by entering the
olfactory neuroepithelium  and
reaching the brain. N. fowleri trophozoites are found in
cerebrospinal fluid (CSF) and tissue, while flagellated forms are found
in CSF. and
reaching the brain. N. fowleri trophozoites are found in
cerebrospinal fluid (CSF) and tissue, while flagellated forms are found
in CSF.
Acanthamoeba spp. and Balamuthia mandrillaris are
opportunistic free-living amebae capable of causing granulomatous amebic
encephalitis (GAE) in individuals with compromised immune systems.
Acanthamoeba spp. have been found in soil; fresh, brackish, and
sea water; sewage; swimming pools; contact lens equipment; medicinal
pools; dental treatment units; dialysis machines; heating, ventilating,
and air conditioning systems; mammalian cell cultures; vegetables; human
nostrils and throats; and human and animal brain, skin, and lung
tissues. B. mandrillaris however, has not been isolated
from the environment but has been isolated from autopsy specimens of
infected humans and animals. Unlike N. fowleri, Acanthamoeba
and Balamuthia have only two stages, cysts
 and trophozoites
and trophozoites  , in their
life cycle. No flagellated stage exists as part of the life cycle.
The trophozoites replicate by mitosis (nuclear membrane does not remain
intact) , in their
life cycle. No flagellated stage exists as part of the life cycle.
The trophozoites replicate by mitosis (nuclear membrane does not remain
intact)  . The
trophozoites are the infective forms and are believed to gain entry into
the body through the lower respiratory tract, ulcerated or broken skin
and invade the central nervous system by hematogenous dissemination . The
trophozoites are the infective forms and are believed to gain entry into
the body through the lower respiratory tract, ulcerated or broken skin
and invade the central nervous system by hematogenous dissemination
 .
Acanthamoeba spp. and Balamuthia mandrillaris cysts and
trophozoites are found in tissue. .
Acanthamoeba spp. and Balamuthia mandrillaris cysts and
trophozoites are found in tissue.
CDC DPDx Parasite Image Library |
| |
A

B

Figure 26 Acanthamoeba sp. keratitis. A: Biopsy showing a cyst; B: cyst, at a larger magnification, with a characteristic shape, in corneal scraping.
CDC |
Acanthameba
Several species of free-living Acanthameba are pathogenic to man. They
normally reside in soil and can infect children who swallow dirt while
playing on the ground. In normal individuals, the infection may cause mild
disease (pharyngitis)
or remain asymptomatic, but in immunodeficient individuals, the organism may
penetrate the esophageal mucosa and reach the brain where it causes Granulomatous
Amebic Encephalitis (figure 26).
Granulomatous Amebic
Encephalitis
This is a rare infection that can affect the brain and disseminate to
the rest of the body. It can affect healthy people but is normally
associated with immunocompromized individuals (organ transplants,
lymphocyte disorders) and patients with diabetes, cancer, liver
cirrhosis, lupus and people who have used antibiotics and steroids
excessively.
Most cases are fatal. The
use of miltefosine is recommended by CDC.
Acanthameba Keratitis
Most cases of this disease in the United States occur in contact lens
users (1 to 33 cases per million). It results from improper storage and
cleaning of lenses in tap water.
|
Summary |
|
Organism |
Transmission |
Disease/symptoms |
Diagnosis |
Treatment |
|
Trypanosoma
brucei
|
Tsetse
fly. |
Sleeping
sickness; cardiac failure. |
Hemoflagellate
in blood or lymph node. |
Blood
stage: Suramin or petamidine isethionate; |
|
T.
cruzi |
Reduvid
(kissing) bug. |
Chagas
disease: megacolon, cardiac failure.
|
Hemoflagellate
in blood or tissue. |
CNS:
melarsoprol
Nifurtimox
and Benzonidazole. |
|
Leishmania
donovani |
Sand
fly |
Visceral leish-maniasis, granulo-matous skin lesions. |
Intracellular
(macrophages) leishmanial bodies.
|
Pentosam;
Pentamidine isethionate. |
|
L.
tropica |
Sand
fly. |
Cutaneous
lesions. |
As for
L. donovani. |
As for
L. donovani. |
|
L.
braziliensis |
Sand
fly |
Mucocutaneous
lesions. |
As for
L. donovani. |
As for
L. donovani. |
|
Plasmodium
falciparum
P. ovale, P. malariae and P. vivax |
Female
anopheline mosquito.
|
Malarial
paroxysm: chills, fever, headache, nausea cycles.
|
Plasmodia
in rbc, typical of the species involved. |
Quinine
derivatives
Proguanil
Lariam |
|
Babesia
microti
|
Tick |
Hemolytic
anemia, Jaundice and fever |
Typical
organism (Maltese cross) in rbc. |
None;
self resolving. |
|
Toxoplasma
gondii |
Oral
from cat fecal material;
or meat
|
Adult:
flu like;
congenital:
abortion, neonatal blindness and neuropathies.
|
Intracellular
(in macrophages) tachyzoites. |
Sulphonamides,
pyemethamine, possibly spiramycin (non-FDA).
|
|
Pneumocystis
jiroveci |
Cough
droplets |
Pneumonia
|
Pneumocystis
in sputum. |
Trimethoprim
and sulphamethoxazole. |
|
|

 |
 Return to the Parasitology Section of Microbiology and Immunology On-line
Return to the Parasitology Section of Microbiology and Immunology On-line
This page last changed on
Monday, February 23, 2015
Page maintained by
Richard Hunt
|


 Figure 1A
Figure 1A

 Figure 2A
Figure 2A  Figure 2D
Figure 2D  Figure 2C
Figure 2C  Figure 3 Tsetse fly. The vector of African trypanosomiasis
© OhioState University, College of Biology
Figure 3 Tsetse fly. The vector of African trypanosomiasis
© OhioState University, College of Biology Figure 6 Chaga's disease: Countries in which American trypanosomiasis is endemic.
WHO
Figure 6 Chaga's disease: Countries in which American trypanosomiasis is endemic.
WHO Figure 7A
Figure 7A 

 Figure 9C
Figure 9C Figure 10D
Figure 10D  Figure 10G
Figure 10G
 Figure 10 F
Figure 10 F
 Figure 10J
Figure 10J 
 Figure 11B
Figure 11B  Figure 11D
Figure 11D  Figure 11F
Figure 11F Figure 12A
Figure 12A  Figure 12D
Figure 12D  Figure 12F
Figure 12F


 Figure
18
Figure
18 Figure 21D
Figure 21D
 Figure 24A
Figure 24A  Figure 24D
Figure 24D
 Figure 25 A
Figure 25 A  Figure 25C
Figure 25C  Figure
25E
Figure
25E 





















































































