|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
IN TURKISH |
VIROLOGY - CHAPTER ELEVEN
HERPES VIRUSES
Dr Richard Hunt
Professor of Pathology, Microbiology and Immunology
University of South Carolina School of Medicine
|
|
En Español |
|
SHQIP - ALBANIAN |
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|
|
|

 |
|
Logo image © Jeffrey Nelson, Rush
University, Chicago, Illinois and
The MicrobeLibrary |
|
|
|
|
| |
|
Mercutio (to Romeo) in
Romeo and Juliet by Shakespeare:
O'er ladies lips, who
straight on kisses dream,
Which oft the angry Mab with blisters plagues,
Because their breaths with sweetmeats tainted are:
Sometime she gallops o’er a courtier’s nose,
And then dreams he of smelling out a suit;
And sometime comes she with a tithe-pig’s tail
Tickling a parson’s nose as a’ lies asleep.... |
|
|
|
 FIGURE 1 Classification of Herpes viruses
FIGURE 1 Classification of Herpes viruses |
Introduction
Herpes viruses are a leading cause of human viral
disease, second only to influenza and cold viruses. They are capable of causing
overt disease or remaining silent for many years only to be reactivated, for
example as shingles. The name herpes comes from the Latin herpes which,
in turn, comes from the Greek word herpein which means to creep. This
reflects the creeping or spreading nature of the skin lesions caused by many
herpes virus types.
There are at least 25 viruses in the family
Herpesviridae (currently divided into three sub-families). Eight or more herpes
virus types are known to infect man frequently (table 1 and 2, figure 1).
|
FIGURE 2
(below)
Herpes virus structure
 Herpes Virus structure. Between the nucleocapsid and the membrane is the
ill-defined tegument
Herpes Virus structure. Between the nucleocapsid and the membrane is the
ill-defined tegument
 Herpes Simplex Virus-1 A-capsid from 400kV Spot-scan Electron
Cryomicroscopy
© 1994 Zhou et al. Baylor
College of Medicine
Herpes Simplex Virus-1 A-capsid from 400kV Spot-scan Electron
Cryomicroscopy
© 1994 Zhou et al. Baylor
College of Medicine
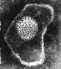 Herpes simplex virus. Negative stain.
Copyright
Dr Linda M Stannard,
University of Cape Town, South Africa, 1995 (used with permsssion)
Herpes simplex virus. Negative stain.
Copyright
Dr Linda M Stannard,
University of Cape Town, South Africa, 1995 (used with permsssion) |
|
TABLE 1
HERPES VIRUS TYPES THAT INFECT HUMANS |
|
Herpes simplex virus Type 1 (HSV-1) |
|
Herpes simplex virus Type 2 (HSV-2) |
|
Epstein Barr virus (EBV) |
|
Cytomegalovirus (CMV) |
|
Varicella Zoster Virus (VZV) |
|
Human herpes virus 6 (exanthum subitum or roseola infantum) |
|
Human herpes virus 8 (Kaposi's sarcoma-associate herpes virus) |
Once a patient has become infected by herpes virus, the infection remains for
life. The initial infection may be followed by latency with subsequent
reactivation. Herpes viruses infect most of the human population and persons
living past middle age usually have antibodies to most of the above herpes
viruses with the exception of HHV-8.
Herpes viruses are classified by their location in
the latent state (table 2).
|
 Liquid-Crystalline, Phage-like Packing of Encapsidated DNA in Herpes
Simplex Virus
(F.P.Booy, W.W.Newcomb,
B.L.Trus, J.C.Brown, T.S.Baker, and A.C.Steven, in CELL, Vol 64 pp
1007-1015, March 8, 1991)
Liquid-Crystalline, Phage-like Packing of Encapsidated DNA in Herpes
Simplex Virus
(F.P.Booy, W.W.Newcomb,
B.L.Trus, J.C.Brown, T.S.Baker, and A.C.Steven, in CELL, Vol 64 pp
1007-1015, March 8, 1991)
 3-D computer reconstruction from cryo-electron micrographs
of herpes simplex virus capsids. Rotating image.
National Institutes of Health
3-D computer reconstruction from cryo-electron micrographs
of herpes simplex virus capsids. Rotating image.
National Institutes of Health
 Herpesvirus (entire particle) solved
by cryo-electron microscopy and image reconstruction MPEG version
Herpesvirus (entire particle) solved
by cryo-electron microscopy and image reconstruction MPEG version
 Herpesviruses have an envelope surrounding an icosahedral
capsid, approximately 100nm in diameter, which contains the dsDNA
genome. When the envelope breaks and collapses away from the
capsid, negatively stained virions have a typical "fried-egg"
appearance.
Copyright
Dr Linda M Stannard,
University of Cape Town, South Africa, 1995 (used with permission)
Herpesviruses have an envelope surrounding an icosahedral
capsid, approximately 100nm in diameter, which contains the dsDNA
genome. When the envelope breaks and collapses away from the
capsid, negatively stained virions have a typical "fried-egg"
appearance.
Copyright
Dr Linda M Stannard,
University of Cape Town, South Africa, 1995 (used with permission) |
|
TABLE 2 -
Properties of Herpes viruses |
|
Human herpes type |
Name |
Sub Family |
Target cell type |
Latency |
Transmission |
|
1 |
Herpes
simplex-1 (HSV-1) |
Alphaherpesvirinae |
Mucoepithelia |
Neuron |
Close contact |
|
2 |
Herpes
simplex-2 (HSV-2) |
Alphaherpesvirinae |
Mucoepithelia |
Neuron |
Close contact
usually sexual |
|
3 |
Varicella
Zoster virus (VZV) |
Alphaherpesvirinae |
Mucoepithelia |
Neuron |
Contact or
respiratory route |
|
4 |
Epstein-Barr
Virus (EBV) |
Gammaherpesvirinae |
B lymphocyte,
epithelia |
B lymphocytes |
Saliva |
|
5 |
Cytomegalovirus (CMV) |
Betaherpesvirinae |
Epithelia,
monocytes, lymphocytes |
Monocytes,
lymphocytes and possibly others |
Contact, blood
transfusions, transplantation, congenital |
|
6 |
Herpes
lymphotropic virus |
Betaherpesvirinae |
T lymphocytes
and others |
T lymphocytes
and others |
Contact,
respiratory route |
|
7 |
Human herpes
virus-7 (HHV-7) |
Betaherpesvirinae |
T lymphocytes
and others |
T lymphocytes
and others |
Unknown |
|
8 |
Human herpes
virus-8 (HHV-8)
Kaposi's sarcoma- associated herpes virus (KSHV) |
Gammaherpesvirinae |
Endothelial
cells |
Unknown |
Exchange of
body fluids? |
|
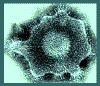 Glycoprotein "spikes" on the HSV surface. Glycoprotein B (gB)
is clearly visualised in clusters of spikes about 10 nm in length. Between
the capsid and the envelope is an ill-defined layer of proteins,
collectively known as the tegument.
Copyright
Dr Linda M Stannard,
University of Cape Town, South Africa, 1995 (used with permission)
Glycoprotein "spikes" on the HSV surface. Glycoprotein B (gB)
is clearly visualised in clusters of spikes about 10 nm in length. Between
the capsid and the envelope is an ill-defined layer of proteins,
collectively known as the tegument.
Copyright
Dr Linda M Stannard,
University of Cape Town, South Africa, 1995 (used with permission)
|
Herpes Virus Structure - General
Envelope
Herpes viruses are enveloped viruses.
They bud from the inner nuclear membrane which has been modified by the
insertion of herpes glycoproteins (in the mature virus, these glycoproteins
determine the cell to be infected because of the availability of the
appropriate receptors). The viral membrane is quite fragile and a virus with
a damaged envelope is not infectious (This means that the virus readily
falls apart and so the virus can only be obtained by direct contact with
mucosal surfaces or secretions of an infected person - it cannot be caught
from toilet seats). Besides drying, the virus is also sensitive to acids,
detergents and organic solvents as might be expected for an virus with a
lipid envelope.
Tegument
The space between the envelope and the
capsid is the tegument. This contains virally-encoded proteins and enzymes
involved in the initiation of replication
Capsid
These viruses have a doughnut shaped
capsomere of about 100-200 nm in diameter with an icosahedral nucleocapsid.
The latter contains 162 capsomeres
Genome
These viruses have double stranded DNA.
The size of the genomes differs with cytomegalovirus having the largest
genome
|
FIGURE 3 (below)
Genomes of herpes viruses
 Genomes of herpes viruses. HSV, VZV and CMV have inverted
repeat sequences. This results in the formation of more than one isomer by
recombination. Because VZV has only two inverted repeats, it can only form
two isomeric forms. Direct repeats do not allow recombination and so
EBV and HHV6 have only one isoform.
Genomes of herpes viruses. HSV, VZV and CMV have inverted
repeat sequences. This results in the formation of more than one isomer by
recombination. Because VZV has only two inverted repeats, it can only form
two isomeric forms. Direct repeats do not allow recombination and so
EBV and HHV6 have only one isoform.
|
Herpes virus replication
i) Binding to the cell surface: As with many
other viruses, cell tropism is determined by the availability of the
correct receptor on the surface of the cell to be infected. The virus
fuses with the cell membrane at ambient pH and so there is the
possibility of syncytia formation between infected cells and therefore
cell to cell transmission even in the presence of neutralizing humoral
antibodies. This means that cell-mediated immunity is important in
suppressing herpes virus infections.
ii) Nucleocapsid enters cytoplasm: The tegument-surrounded nucleocapsid
is carried to the nuclear membrane where the nucleocapsid binds. The DNA
genome then enters the nucleus.
iii) Transcription: This is a very complex process, as might be expected
from the large size of genome. There are three classes of proteins that
need to be made for the production of a mature virus.
- Alpha proteins: These are the
immediate-early proteins. They are involved in transcriptional
regulation and are not found in the mature virion. They are also
involved in the control of beta protein synthesis (figure 4).
|
|
MOVIE
Replication of herpes
(requires Flash)
|
|
 Figure 4
Figure 4
Herpes virus gene expression
Expression of immediate early, early and late genes of
herpesviruses
 Figure 5
Figure 5
Maturation of herpes viruses
Stages in the exocytosis of herpes virus from the nucleus, in which the
virus core is assembled, to the plasma membrane
|
- Beta proteins. These are the early
proteins and are involved also in DNA replication (they include the DNA
polymerase and transcription factors). Only a few copies of DNA
polymerase need to be made for replication to occur (figure 4).
- Gamma proteins. These are the late
proteins and are structural components of the virus. The synthesis of
gamma proteins is initiated after the start of DNA synthesis (figure 4).
iv) RNA transcription: The herpes DNA is
transcribed to RNA by a cellular enzyme (DNA-dependent RNA polymerase I).
However, the transcription of the various genes is dependent on both nuclear
factors of the cell AND proteins encoded by the virus. This control of viral
mRNA, and therefore, viral protein, synthesis determines whether infection will
result in the production of new virus particles and cell death (a lytic
infection), persistent shedding of virus (persistent infection) or latency.
Whether latency occurs is the property of the host cell, that is some cells do
not allow the replication of viral DNA. If the cell permits progression beyond
the immediate early genes, a lytic infection will ensue.
v) DNA synthesis: Herpes viruses encode their own
DNA-dependent DNA polymerase. In addition, some herpes viruses encode enzymes
(such as thymidine kinase) that allow the virus to grow in non-dividing cells
that do not therefore contain the precursors of DNA synthesis. Without this
enzyme, neurotropic herpes viruses could not replicate because of the low
amounts of certain DNA precursors in nerve cells.
vi) Assembly: Nucleocapsids are assembled in the
nucleus and are filled with DNA They then bud through the double nuclear
membrane and leave the cell via the exocytosis pathway or they may bud through
another cell membrane such as the plasma membrane (figure 5).
|
|
|
Herpes simplex Virus (HSV)
(figure 6)
These are very large viruses and their genome
encodes at least 80 proteins. Many of these proteins (about half) are not
directly involved in the virus structure or controlling its replication but
function in the interaction with the host cell or the immune response of the
host.
There are two types, HSV-1 and HSV-2 with very
similar characteristics
The genome of HSV also encodes a number of enzymes:
- DNA-dependent DNA polymerase
- Thymidine kinase (phosphorylates thymidine and
other nucleosides)
- Ribonucleotide reductase (converts
ribonucleotides to deoxyribonucleotide
- Serine-protease (convert a scaffolding protein
to its final form) (figure 7)
The genome encodes 11 surface glycoproteins. These
are involved in:
Attachment (gB, gC, gD and gH)
Fusion of the viral membrane with that of the
host cell (gB)
Immune escape and other functions (gC, gE and gI).
An example of the immune escape function is gC which binds complement C3
protein and thus depletes it from the host's serum and inhibits
complement-mediated reactions. The virus gE and gI proteins can also bind
IgG via the Fc portion of the immunoglobulin. This coats the virus with
immunoglobulin and hides it from the immune system.
|
FIGURE 6
Herpes simplex virus - Electron micrographs
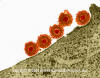 Herpes Simplex Virus (TEM x169,920)
Copyright
Dr Dennis Kunkel (used with
permission)
Herpes Simplex Virus (TEM x169,920)
Copyright
Dr Dennis Kunkel (used with
permission)
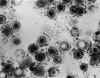 Transmission electron micrograph of herpes simplex virus. Some
nucleocapsids are empty, as shown by penetration of electron-dense
stain.
CDC/Dr. Erskine Palmer
Transmission electron micrograph of herpes simplex virus. Some
nucleocapsids are empty, as shown by penetration of electron-dense
stain.
CDC/Dr. Erskine Palmer
|
 Figure 7
Figure 7
The serine protease of herpes viruses. Click on the image at left to link to
an interactive structure of the cytomegalovirus protease. This protease is
essential for the production of mature infectious virions, as it performs
proteolytic processing of a viral assembly protein precursor. (Requires a
Chime plug-in. Get Chime
here)
|
HSV replication
Almost any human cell type can be infected by
HSV. In many cells, such as endothelial cells and fibroblasts, infection is
lytic but neurones normally support a latent infection.
Binding
The initial step of the interaction of virus with the cell is binding to
the proteoglycan, heparan sulfate. This molecule is found on the
surfaces of many cells.
Fusion
After binding, the virus fuses directly with the plasma membrane (no
entry into low pH endosomes/lysosomes is necessary). After fusion
occurs, the virus releases some proteins into the cytoplasm. These
include some toxins, a protein kinase and a gene transcription
initiator.
Protein synthesis
Immediate early genes are first transcribed which promote transcription
of early genes. If the infection is to be latent, the only mRNAs that
are made are the latency-associated transcripts. The early gene products
include the DNA polymerase plus enzymes that degrade cellular mRNA and
proteins. If early and late proteins are made, the cell is set on a
route to lysis.
As noted above, only a few DNA polymerase
proteins need to be made for replication of viral DNA. At first,
circular concatomers are made but then synthesis switches to linear
chains of individual molecules that are cleaved into monomers. This
occurs by a rolling circle mechanism (see lecture). Late genes are now
transcribed in large amounts, probably triggered by the synthesis of
DNA. They are translated in the cytoplasm and transported back into the
nucleus where they are assembled into the procapsid. The latter is
filled with viral DNA.
Glycoprotein
synthesis
All glycoproteins are made in the rough
endoplasmic reticulum where they receive high mannose sugar chains. The
glycoproteins are moved to the nuclear membrane, probably by a process
of diffusion since the membrane of the endoplasmic reticulum is
continuous with the outer nuclear membrane. How the proteins get around
the nuclear pore is unknown. The nucleocapsids now bud through the
nuclear membrane via areas in which the viral proteins are concentrated.
In some way, the virus enters the exocytotic pathway since it is
modified in the Golgi body where is receives sugar chains that are
characteristic of Golgi-processed proteins (that is, they contain
galactose and sialic acid).
Release of virus
Several pathways seem to occur. The virus can proceed along the
exocytotic pathway or it can enter the cytoplasm and be released by cell
lysis. It also appears to be able to pass through intercellular
junctions and thereby spread from cell to cell.
|
| |
Pathogenesis
The hallmark of herpes infection is the ability
to infect epithelial mucosal cells or lymphocytes. The virus then travels up
peripheral nerves to a nucleated neurone where it may stay for years
followed by reactivation. A reddened area gives rise to a macula which
crusts to form a papula. The fluid in this blister is full of virus. As long
as the virus is kept moist it can remain infectious
Herpes simplex 1 and 2 can infect both humans
and other animals but only humans show symptoms of disease. As noted above,
HSV-1 and HSV-2 first infect cells of the mucoepithelia or enter through
wounds. They then frequently set up latent infections in neuronal cells. The
site of the initial infection depends on the way in which the patient
acquires the virus. It is often noted that HSV-1 causes infections above the
waist and HSV-2 below the waist but this reflects the mode of transmission
rather than any intrinsic property of the virus. Both types of HSV can also
persistently infect macrophages and lymphocytes. The presence of the virus
is often indicated by the formation of syncytia and Cowdry type A inclusion
bodies in the nucleus. Once epithelial cells are infected, there is
replication of the virus around the lesion and entry into the innervating
neurone. The virus travels along the neurone (by a process called retrograde
transport) to the ganglion. In the case of herpes infections of the oral
mucosa, the virus goes to the trigeminal ganglia whereas infections of the
genital mucosa lead the virus entering the sacral ganglia. The virus can
also travel in the opposite direction to arrive at the mucosa that was
initially infected. Vesicles containing infectious virus are formed on the
muscosa and the virus spreads. The vesicle heals and there is usually no
scar as a result.
The immune response to HSV 1 and 2
As might be expected, both the cellular
and humoral arms of the immune response are involved. Interferon is
important in limiting the initial infection and natural killer cells are
also involved at this stage. Cytotoxic T cells and macrophages form the
cellular arm of the response and kill infected cells. The humoral arm of the
response (usually antibodies against surface glycoproteins) leads to
neutralization. As noted above, the virus can escape the immune system by
coating itself with IgG via Fc receptors and complement receptors. The virus
can also spread from one cell to another without entering the extracellular
space and coming in contact with humoral antibodies. This means that
cell-mediated responses are vital in controlling herpes infections. The cell
mediated and inflammatory response lead to some of the disease symptoms.
|
| |
Latency
The virus particles can infect
neurones and since only immediate early proteins are made, there is no
cytopathic effect (although the presence of the virus can be detected by
techniques such as immunofluorescence microscopy using antibodies
against the immediate early proteins). Breakage of latency can occur in
these cells and the virus travels back down the nerve axon. Lesions now
occur at the dermatome, that is the area of skin innervated by a single
posterior spinal nerve. This means that recurrence of infection (and
therefore symptoms) occurs at the same site as the initial infection.
There are several agents that seem to trigger recurrence, most of which
are stress-related. It also appears that exposure to strong sunlight and
perhaps fever can lead to recurrence. These factors may cause some
degree of immune suppression that leads to renewal of virus
proliferation in the nerve cell. Recurrent infections are usually less
pronounced than the primary infection and resolve more rapidly.
|
|
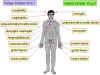 Figure 8
Figure 8
Site at which HSV-1 and HSV-2 cause disease in humans
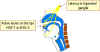 Figure 9A
Figure 9A
Herpes simplex virus can set up a primary infection in the lips, move to
the trigeminal ganglion where it can remain latent. Virus can
subsequently reactivate, move to the original site of infection and
result in cold sores
|
Epidemiology
HSV 1 and 2 infections are life-long and
although latency is soon set up, the infected patient can infect others as a
result of recurrence. The virus is found in the lesions on the skin but can
also be present in a variety of body fluids including saliva and vaginal
secretions. Despite the apparent above the waist/below the waist
rule, both types of HSV can infect oral or genital mucosa depending on the
regions of contact (figure 8). However, HSV-1 is usually spread mouth to
mouth (kissing or the use of utensils contaminated with saliva) or by
transfer of infectious virus to the hands after which the virus may enter
the body via any wound or through the eyes. A large proportion of the
population has evidence of HSV-1 infection as judged by antibodies. As a
result of poor hygiene in underdeveloped countries, HSV-1 antibodies are
found in more than 90% of children.
HSV-2 is normally spread sexually and is found
in the anus, rectum and upper alimentary tract as well as the genital area.
In addition, as noted above, an infant can be infected at birth by a
genitally-infected mother. The infant can also be infected in utero
if the mother's infection spreads. Because of the infant's underdeveloped
immune system, the resulting infection can be very severe and sometimes lead
to death.
Anyone who comes in contact with fluid
containing infectious virus is at risk. There is a disease that affects
health care workers called herpetic whitlow that results in lesions on the
fingers (it can be caused by either type of HSV). As might be expected,
HSV-2 infections are more prevalent later in life as the number of sexual
contacts increases. Thus, the lowest rates of infection are found in
children and the highest rates in prostitutes among whom as many as 80% are
infected with HSV-2.
|
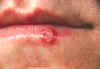 Figure 9B
Figure 9B
Herpes simplex lesion of lower lip, second day after onset.
CDC/Dr. Herrmann
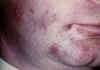 Figure 9C
Figure 9C
Herpes simplex 1: Cold sores
©
Bristol Biomedical Image Archive.
Used with permission
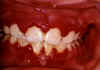 Figure 9D
Figure 9D
Herpetic gingivitis
©
Bristol Biomedical Image Archive.
Used with permission
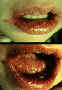 Figure 9E
Figure 9E
Gingivostomatitis looks different from a cold sore, occurs only once and
is usually so mild as to go unnoticed.
©
Australian Herpes Management Forum
|
Diseases caused by Herpes Simplex
Viruses
Herpes simplex 1 and 2 are frequently benign
but can also cause severe disease. In each case, the initial lesion looks
the same. A clear vesicle containing infectious virus with a base of red (erythomatous)
lesion at the base of the vesicle. This if often referred to as a 'dewdrop
on a rose petal'. From this pus-containing (pustular), encrusted lesions and
ulcers may develop.
Oral herpes - Cold
sores
As already stated, this can be the result of an
HSV-1 or an HSV-2 infection. Because of the association of HSV-2 with
sexual transmission, infections in children are usually the result of
HSV-1. In primary herpetic gingivostomatitis , the typical clear lesions
first develop followed by ulcers that have a white appearance. The
infection, often initially on the lips spreads to all parts of the mouth
and pharynx. Reactivation from the trigeminal ganglia can result in what
are known as cold sores. Herpes pharyngitis is often associated with
other viral infections of the upper respiratory tract. The disease is
more severe in immunosuppressed people such as AIDS patients (figure 9)
Herpes keratitis
This is an infection of the eye and is primarily caused by HSV-1. It can
be recurrent and may lead to blindness. It is a leading cause of corneal
blindness in the United States.
|
|
|
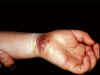 Figure 10
Figure 10
Herpetic whitlow on the wrist
©
Bristol Biomedical Image Archive.
Used with permission
|
Herpes whitlow
This disease of persons who come in manual contact with
herpes-infected body secretions can be caused by either type of HSV
and enters the body via small wounds on the hands or wrists. It can
also be caused by transfer of HSV-2 from genitals to the hands
(figure 10).
Herpes
gladiatorum
This is contracted by wrestlers. It
apparently spreads by direct contact from skin lesions on one
wrestler to his/her opponent, and usually appears in the head and
neck region (which are frequently sites of contact in wrestling
holds). Oddly, the lesions are more often on the right side of the
body (perhaps because most wrestlers are right handed). It is also
seen in other contact sports such as rugby where it is known as
scrum pox (Herpes Rugbeiorum).
|
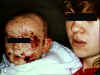 Figure 11
Figure 11
Mother with cold sore on lip holding baby with eczema herpeticum
©
Bristol Biomedical Image Archive.
Used with permission
|
Eczema herpeticum
This is found in children with active eczema, preexisting atopic
dermatitis, and can spread over the skin at the site of eczema
lesions (figure 11). The virus can spread to other organs such as
the liver and adrenals. A similar disease may also be caused by
vaccinia (eczema vaccinatum).
|
|
|
Genital herpes
Genital herpes is usually the result of HSV-2 with about 10% of cases being
the result of HSV-1. Primary infection is often asymptomatic but many painful
lesions can develop on the glans or shaft of the penis in men and on the vulva,
vagina, cervix and perianal region of women (figure 12). In both sexes, the urethra can be
involved. In women, the infection may be accompanied by vaginal discharge.
Genital herpes infections, which involve a transient viremia, can be accompanied
by a variety of symptoms including fever, myalgia, glandular inflammation of the
groin area (inguinal
adenitis). Secondary episodes of genital herpes, which
occur as a result of reactivation of virus in the sacral ganglion, are
frequently less severe (and last a shorter time) than the first episode.
Recurrent episodes seem usually to result from a primary HSV-2 infection.
Patients who are about to experience a recurrence usually first experience a
prodrome in which there is a burning sensation in the area that is about to
erupt. Some patients have only infrequent recurrences but others experience
recurrences as often as every 14-21 days. Whether there is an apparent active
disease or not, an infected patient remains infectious without overt symptoms.
Clearly, these persons are very important in the spread of herpes infection.
HSV proctitis
This is an inflammation of the rectum and the anus (figure 13).
HSV Encephalitis
This is usually the result of an HSV-1 infection and is the most common
sporadic viral encephalitis. HSV encephalitis is a febrile disease and may
result in damage to one of the temporal lobes. As a result there is blood in the
spinal fluid and the patient experiences neurological symptoms such as seizures.
The disease can be fatal but in the US there are fewer than 1000 cases per year.
HSV Meningitis
This is the result of an HSV-2 infection. The symptoms seem to resolve
spontaneously.
|
 Figure 12A
Figure 12A
Genital herpes on the penis
©
Australian Herpes Management Forum
 Figure 12B
Figure 12B
Genital herpes on the penis
©
Australian Herpes Management Forum
 Figure
12C Figure
12C
Classical primary genital herpes affecting the vulva. This clinical picture is seen in a minority of
cases ©
Australian Herpes Management Forum
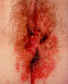 Figure 13
Figure 13
Misdiagnosed perianal herpes. This woman also has severe secondary
Staphylococcal infection
©
Australian Herpes Management Forum |
|
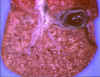 Figure 14
Figure 14
Neonatal herpes simplex infection of the liver © Bristol
Biomedical Image Archive. Used with permission
|
HSV infection of neonates
This results from HSV-2 and is often fatal, although such infections are
rare. Infection is especially possible if the mother is shedding virus at the
time of delivery. Thus prospective mothers should avoid contracting herpes
during pregnancy. A first episode of HSV-2 infection during pregnancy creates a
greater risk of transmission to the newborn. If a woman has active genital
herpes at delivery, a cesarean-section delivery is usually performed. The virus
can either be obtained in utero or during birth with the latter being
more common. Because the neonate has an underdeveloped immune system, the virus
can spread rapidly to many peripheral organs (e.g. lungs and liver) and can
infect the central nervous system (figure 14).
|
|
 Figure 15A
Figure 15A
Herpes simplex 1, Human Plaque Assay. Cells grown on African green
monkey cells. Phase contrast image. © Bristol
Biomedical Image Archive. Used with permission
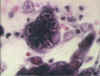 Figure 15B
Figure 15B
Herpes simplex 1: Histological stain. Note the multinucleate cell with
dark staining inclusions. ©
Bristol Biomedical Image Archive. Used with permission
|
Diagnosis of HSV Infections
Cells may be obtained from the base of the lesion (called a Tzank smear) and
histochemistry performed. Since a characteristic of herpes virus is fusion at
neutral pH, infected cells can fuse forming syncytia. These can be seen in the
smears as multinucleated giant cells and contain Cowdry type A inclusion bodies
(figure 15).
The cells can also be stained with specific antibodies in an immunofluorescence
test and it is also possible to detect viral DNA by in situ
hybridization. Type-specific antibodies can distinguish between HSV-1 and HSV-2.
Virus can be isolated from biopsy specimens, that is from the lesions, and
grown on tissue culture cells where it forms characteristic cytopathic effects
(plaque) including multinucleated cells (figure 15). The presence of anti-HSV antibodies in
the patient can be used to form a diagnosis of the primary infection but
recurrence is not usually accompanied by a rise in antibody levels.
HSV chemotherapy
There are a variety of nucleoside analog drugs used to treat herpes
infections, many of which are of high specificity since they take advantage of
the activation of the drug by a viral enzyme, thymidine kinase (see chemotherapy
section). The fact that the drug is only activated in herpes-infected cells
(because only here is the rather specific viral thymidine kinase expressed)
means that these drugs show few side effects.
The best known of the nucleoside analogs is acycloguanosine (acyclovir) but
there are other approved drugs including famciclovir and valacyclovir. All of
these nucleoside analogs suffer from the appearance of resistant herpes mutants
although resistant strains of the virus are usually less virulent than the wild
type. It should be noted that these drugs act against the replicating virus
(they are incorporated into the DNA as it is copied) and therefore they are
ineffective against latent virus.
Since once the virus infects, the patient has it for life, the best option is
to avoid infection by not coming in contact with the virus. This is particularly
important for health care providers. However, this is not always possible as
many patients with active viral replication are asymptomatic. Patients with
genital herpes should avoid intercourse when they have the prodromal itching
symptoms or an active lesion.
|
|
|
Varicella-Zoster Virus (also known as Herpes Zoster Virus,
Human Herpes Virus-3)
(figure 16)
Zoster means girdle from the characteristic rash that forms a belt around the
thorax in many patients (figure 18). The structure of Varicella virus is very similar to
Herpes Simplex virus although the genome is somewhat smaller
Diseases caused by Varicella-Zoster virus
This virus causes two major diseases, chicken-pox (Varicella),
usually in childhood, and shingles, later in life. Shingles (Zoster) is a
reactivation of an earlier varicella infection.
Chicken Pox
This virus is highly infectious (figure 19) and
even if we do not remember getting it, more than 90% of the population of the US
has antibodies against varicella proteins. In the household of an infected
patient, 90% of contacts who have hitherto not had the disease will get it
(unless vaccinated). It is spread by respiratory aerosols or direct contact with
skin lesions. As with HSV, infection is via mucosa, this time in the respiratory
tract.
During the 10-12 day prodromal stage, the virus in
the respiratory mucosa infects macrophages and pneumocytes. At this stage, there
are no symptoms. The virus spreads from the lungs to lymphocytes and monocytes
and to the reticulo-endothelial system. Here, at about 5 days, a second viremia
occurs and the virus travels to the skin, mouth, conjunctiva, respiratory tract
and, indeed, to epithelial sites throughout the body. Here the virus leaves the
blood vessels and first infects sub-epithelial sites and then epithelial sites
forming papulae containing multinucleated cells with intracellular inclusions.
The virus reaches the surface and is shed to the exterior of the respiratory
tract about 12-14 days after the initial infection. It takes a little longer (a
few days) for the virus to reach the surface of the skin when the characteristic
papulae (rash) appear. At this stage the patient will likely have a fever for a
few days (up to 39 degrees). There are various periods between the initial
infection and the occurrence of the papulae that are diagnostic of chicken pox
but the average is about two weeks with range of 10 to 23 days (figure 17).
Spreading of the disease can be from virus in the respiratory tract (by a cough)
or from contact with ruptured papulae on the skin containing infectious virus.
Thus the contagious period starts at about 12-14 days after the initial
infection.
For some reason, the rash is most pronounced on the
face, scalp and trunk and less on the limbs. The disease is more severe in older
children and adults. This is particularly the case in immunocompromised patients
(AIDS, transplantation etc) where the disease may linger for several weeks and
the fever may be more pronounced. The spread of the virus may lead to problems
in the lungs, liver and to meningitis. In this case mortality may be up to 20%.
Complications
Pneumonia can be associated with a varicella infection (about 15% of adult
patients) and may be fatal.
Although most children recover rapidly from the disease, there are some
complications. These include fulminant encephalitis and cerebellar ataxia. It is
possible that some of these complications may be Reyes syndrome. It has been
suggested that the latter may be cause by aspirin used in chicken pox
infections. Other rare complications of chicken pox are traverse myelitis,
Guillian Barre syndrome and aseptic meningitis.
Congential Varicella syndrome
Major problems may be caused by infection in utero
during the first
trimester. This is congenital varicella syndrome which leads to scarring of the
skin of the limbs, damage to the lens, retina and brain and microphthalmia.
Infection of the mother, who presumably has not previously been infected and
therefore does not have anti-varicella antibodies, at around the time of birth
can lead to the infection of the infant. Since the infant will not have maternal
antibodies against varicella and has immature cell-mediated immunity, it may
succumb to the disease with a mortality rate of up to 35%. If the mother becomes
infected near to term, both she (before delivery) and her infant (immediately
after delivery) should be treated with varicella immune globulin. Most infants,
however, get maternal antibodies trans-placentally and are protected from the
disease.
|
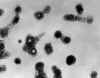 Figure 16A
Figure 16A
Transmission electron micrograph of varicella- zoster virions from vesicle fluid of patient with chickenpox
CDC/Dr. Erskine Palmer
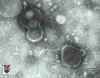 Figure 16B
Figure 16B
Negative stain of varicella zoster virus
© Dr S.
McNulty, Queens University, Belfast. Image must not be used for commercial purpose without the consent of the copyright owners.

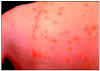 Figure 17A
Figure 17A
This person has chickenpox rash. Some of the sores are red spots and some are blisters.
The red spots will become blisters and new red spots will form CDC
 Figure 17B
Figure 17B
Each spot starts as a 2-4 mm diameter red papule which develops an irregular outline (rose petal) as a
small vesicle appears on the surface. This 'dew drop on a rose petal' appearance is very characteristic of
chickenpox. ©
Australian Herpes Management Forum
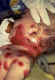 Figure 17C
Figure 17C
This is a classic case of chickenpox of the newborn. The infant contracted
chickenpox at birth from her infected mother.
A severe skin infection has developed on the face and neck and, without treatment, this
infection could spread throughout the
body and cause serious illness or even death
Courtesy of the American Academy of Pediatrics, Pennsylvania
chapter - Immunization Action Coalition
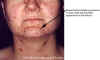
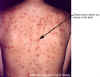 Figure 17D
Figure 17D
Thirty year old female with chicken pox. There
is a general rash over the entire back.
Images © Lewis Tomalty, Queens University, Kingston, Ontario K7L 3N6 Canada
and The MicrobeLibrary |
| |
|
|
|
Shingles
After the infectious period, the virus may migrate to the ganglia associated
with areas in which the virus is actively replicated. The virus may then be
reactivated under stress or with immune suppression. This usually occurs later
in life. The recurrence of varicella replication is accompanied by severe
radicular pain (figure 18) in discrete areas, those innervated by the nerve in which latent
infection has occurred. A few days later chicken pox-like lesions (figure 18) occur in
restricted areas (dermatome) that are innervated by a single ganglion. New
lesions may appear in adjacent dermatomes and even further afield. Reactivation
can affect the eye via the trigeminal nerve (uveitis, keratitis, conjunctivitis,
ophthalmoplegia, iritis) and the brain via the cranial nerve VII and VIII
(Bell's
Palsy and Ramsay-Hunt syndrome (figure 18)). The skin lesions are somewhat different from
those in chicken pox, being restricted to small areas of the skin, usually in
the thorax (figure 18). They are small and close together. They are maculopapular with an
erythematous base and usually heal in about two weeks. Reactivation can lead to
chronic burning or itching pain called post-herpetic neuralgia which is seen
primarily in the elderly. The pain may last well after the rash has healed (even
months or years). Often associated with post-herpetic neuralgia is increased
sensitivity to touch (hyperesthesia).
Patients with AIDS often exhibit multi-dermatomal recurrence of varicella
infection. There is also a chronic verricous form in some AIDS patients.
|
|
 Figure 18A
Figure 18A
Typical isolated rash in shingles
CDC
 Figure 18C
Figure 18C
In severe cases of shingles, the lesions coalesce, forming a disfiguring carpet of scabs and sometimes the rash leaves permanent
scars
© Australian Herpes Management Forum
|
 Figure 18B
Figure 18B
Shingles affecting the left side of the trunk
©
Australian Herpes Management Forum
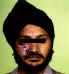
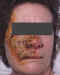 Figure 18D
Figure 18D
Recurrent varicella zoster on the right side of the face
© Bristol
Biomedical Image Archive
 Figure
18E Figure
18E
Severe atypical episode of shingles affecting the trunk of a person with impaired
immunity. Note that the distribution of the lesions resembles a 'sword
belt'. Hence the name zoster
© Australian Herpes Management Forum
|
 Figure 18F
Figure 18F
Disseminated lesions affecting multiple dermatomes
© Australian Herpes Management Forum |
 Figure 18G
Figure 18G
Facial shingles. The ophthalmic division of the trigeminal nerve is the dermatome involved.
© Australian Herpes Management Forum
 Figure 18H
Figure 18H
Shingles affecting the right L1 dermatome
©
Australian Herpes Management Forum
|
 Figure 18I
Figure 18I
Ramsay-Hunt syndrome affecting the ear showing blistering of the
external ear canal
©
Australian Herpes Management Forum |
 Figure 18J
Figure 18J
Ramsay-Hunt syndrome causing a right-sided facial palsy. Paralysis is more obvious in cases of shingles involving the
face. It is caused by an extension of the disease process to motor regions of the spinal
cord or brainstem. In a minority of cases, the areas of paralysis and rash do not
coincide. For instance, rash on the neck and lower part of the face, involving the
trigeminal and cervical nerves, may be associated with paralysis of the facial nerve and
loss of taste. This distribution of rash and combination of motor and sensory symptoms
cannot be explained by involvement of a single nerve ganglion or a mixed motor and
sensory nerve trunk. Rather, it must be the result of a wider, if still local, spread of virus
in the central nervous system.
©
Australian Herpes Management Forum
|
 Figure 18K
Figure 18K
The most common and widely feared complication of shingles is persistence of pain in
the affected area of the body after the rash has healed. This is often called post-herpetic
neuralgia, which may be very severe and prolonged, particularly in older patients.
Unfortunately it can be very resistant to treatment but, by treating shingles with an
antiviral agent within 3 days of the rash appearing, it may be possible either to reduce
the likelihood of developing prolonged pain or, put another way, reduce the overall
duration of pain associated with the condition.
© Australian Herpes Management Forum
 Figure 19
Figure 19
Varicella cases and states reporting, United States, 1972-1996.
CDC/Barbara Rice
|
Diagnosis
Both chicken pox and shingles are diagnosed by their characteristic
appearance but a definitive diagnosis can be made by culture of the virus from
the lesions (a difficult procedure) followed by detection of specific antigens.
The characteristic appearance of cells in biopsy specimens of skin lesions can
also be used.
Treatment
As with HSV, acyclovir (or other nucleoside analogs) can be useful,
particular in preventing dissemination in immunosuppressed patients. Varicella
immunoglobulin can also be used. Normally, however, only supportive care is used
in children who quickly recover if they mount an adequate cell-mediated
response.
Vaccine
There is a live attenuated vaccine virus and this is used in the United
States. It leads to antibody production and cell-mediated immunity. It can be
used post-exposure.
Epstein-Barr Virus
Epstein-Barr virus is the causative agent of
Burkitt's
lymphoma in Africa, nasal pharyngeal carcinoma in the orient and infectious
mononucleosis in the west. It was first discovered as the causative agent of
Burkitt's lymphoma and it was later
found that patients with infectious mononucleosis have antibodies that react
with Burkitt's lymphoma cells.
Receptors for the virus
The virus only infects a small number of cell types that express the receptor
for complement C3d component (CR2 or CD21). These are certain epithelial cells (oro-
and naso-pharynx) and B lymphocytes. This explains the cellular tropism of the
virus.
Semi-permissive replication
B lymphocytes are only semi-permissive for replication of the virus and
infection may either be latent or the cells may be stimulated and transformed by
the virus. When lymphocytes are latently infected the cell contains a few
unintegrated copies (episomes) of the virus genome which are replicated every
time the cell divides. In this case the early immediate genes are expressed
including the EBV nuclear antigens. In addition, two latent membrane proteins, a
protein designated LP (a DNA-binding protein) and two small RNA molecules are
expressed. The membrane proteins are oncogenes.
Permissive replication
In contrast, epithelial cells permit complete lytic replication of the virus.
Epithelial cells allow the expression of the ZEBRA protein which activates early
genes resulting in expression of the polymerase and DNA replication.
Subsequently, capsid proteins and the membrane glycoproteins are made.
Pathogenesis
Transformation of B cells
The virus is replicated in pharyngeal epithelial cells, shed into the saliva
and is taken up by CD21+ B lymphocytes. These cells are normally short-lived,
dying by apoptosis. This is a natural process that allows cells to be generated
for a particular process and then removed when no longer needed. Although B
cells do not show any histological alterations as a result of EBV infection,
they are stimulated to divide and are protected from undergoing apoptosis; thus,
the cell becomes transformed and high levels of monocytes are seen in the
bloodstream. Transformation of the B cell changes the interaction of the cell
with other components of the immune system. HLA markers, CD23 blast antigen and
certain adhesion proteins are expressed. The presence of the virus results in
the expression of an analog of interleukin-10 (IL-10) which inhibits gamma
interferon secretion. This results in the inhibition of T cell responses and
promotes growth of the B cells and IgG secretion. The virus also causes the
cells to produce other cytokines including IL-5 and IL-6.
Burkitt's lymphoma
The association between Epstein-Barr virus and Burkitt's
lymphoma has long been established. This is a tumor of the jaw and face found
in children (figure 20). The tumor cells show evidence of EBV DNA and tumor antigens and
patients show a much higher level of anti-EBV antibodies than other members of
the population. Tumor cells are monoclonal and show a very characteristic
translocation between chromosomes 8 and 14. This brings the c-myc next to the
gene for the immunoglobulin heavy chain. As a result, the oncogene is next to
the promotor for a gene that is highly expressed in B lymphocytes resulting is
elevated transcription of c-myc. It should be noted that this translocation is
not seen in infectious mononucleosis patients. Biopsy tissue shows large
multinucleated cells (figure 21). Further evidence that
implicates EBV in Burkitt's lymphoma
is the observation that EBV can transform B lymphocytes in culture and can
produce B cell lymphomas in primates.
This lymphoma is endemic in equatorial Africa but only occurs rarely
elsewhere. Why this is so is unclear but there is probably a genetic reason
possibly involving an association with malaria. Persons who are resistant to
malaria appear to be susceptible to progression to the lymphoma.
|
|
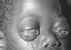 Figure 20
Figure 20
Burkitt's Lymphoma The Johns Hopkins Autopsy Resource
(JHAR) Image Archive
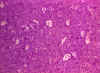 Figure 21
Figure 21
Burkitt's lymphoma histological stain. Notice the large multinucleated
cells
© Bristol Biomedical Image Archive. Used with
permission
|
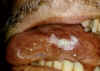 Figure 22
Figure 22
Oral hairy leukoplakia of tongue in AIDS
©
Bristol
Biomedical Image Archive
|
Nasopharyngeal cancer
This disease, which occurs in a number of areas (south China, Alaska,
Tunisia, east Africa), is also associated with EBV. There may be a genetic
predisposition to the development of EBV cancers in these populations or there
may be an environmental cofactor involved. The disease is a tumor of the
epithelium of the upper respiratory tract and the cells contain EBV DNA. The
titer of anti-EBV antibodies alter as the tumor progresses.
Oral hairy leukoplakia
This EBV-associated disease results in lesions in the mouth and has
increased in frequency recently as it is an opportunistic infection of
HIV-infected patients (figure 22).
|
|
|
Infectious mononucleosis
The primary infection is often asymptomatic but the patient may shed
infectious virus for many years. Some patients develop infectious mononucleosis
after 1-2 months of infection. The disease is characterized by malaise,
lymphadenopathy, tonsillitis (figure 23), enlarged spleen and liver and fever. The fever may
persist for more than a week. There may also be a rash. The severity of disease
often depends on age (with younger patients resolving the disease more quickly)
and resolution usually occurs in 1 to 4 weeks.
Although infectious mononucleosis is usually benign, there may be
complications. These include neurological disorders such as meningitis,
encephalitis, myelitis and Guillain-Barrè syndrome. Secondary infections,
autoimmune hemolytic anemia,
thrombocytopenia,
agranulocytosis, aplastic anemia
may also occur. As noted above a chronic syndrome may also occur. The symptoms
are similar to those reported for chronic fatigue syndrome (headaches, sore
throat and low fever) but EBV is probably not the cause of chronic fatigue
syndrome.
|
|
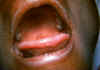 Figure 23A
Figure 23A
Tongue and palate of patient with infectious mononucleosis.
CDC/Emory U./Dr. Sellers
|
In infectious mononucleosis, infected B cells are also transformed. The
infected B cells proliferate and activate suppressor CD8 T cells. These T cells
differ from normal T cells in appearance and are known as Downey cells. The T
cells increase in number in the circulation and may account for up to 80% of the
white blood cells. This T cell response results in enlarged lymph glands (and
enlarged liver and spleen). The activation of the T cells limits the
proliferation of B cells and the disease resolves.
If cell mediated immunity is suppressed, resolution of the disease may not
occur. Uncontrolled viral replication may lead to a severe syndrome with B cell
lymphoproliferation, leukopenia and lymphoma. In patients with T cell deficiency
X-linked lymphoproliferative disorder may occur. Transplant patients and AIDS
patients who are also immunosuppressed may exhibit post-transplant
lymphoproliferative disorder
|
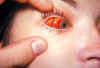 Figure 23B
Figure 23B
A conjunctival hemorrhage of the right eye of this patient with infectious
mononucleosis. At times non-infectious conjunctivitis, as well as other
corneal abnormalities may manifest themselves due to the body’s systemic
response to viral infections such as infectious mononucleosis, or
Epstein-Barr Virus. CDC/Dr.
Thomas F. Sellers/Emory University
 Figure 23C
Figure 23C
Leukemia cells that contain Epstein
Barr virus using a FA staining technique
CDC/Dr.
Paul M. Feorino
|
Epidemiology
A large proportion of the population (90-95%) is infected with Epstein-Barr
virus and these people, although usually asymptomatic, will shed the virus from
time to time throughout life. The virus is spread by close contact (kissing
disease). Infection is associated with socioeconomic factors and in developing
countries, seropositivity is observed at an earlier age than in developed
countries. Up to 80% of students entering college in the US are seropositive for
the virus and many of those that are negative will become positive while at
college. The virus can also be spread by blood transfusion.
Diagnosis
In infectious mononucleosis, blood smears show the atypical lymphocytes
(Downey cells). There are also serological tests available. Heterophile
antibodies are produced by the proliferating B cells and these include an IgM
that interacts with Paul-Bunnell antigen on sheep red blood cells.
Treatment
Unlike herpes simplex virus, there are no drugs available to treat
Epstein-Barr virus. This may reflect the absence of a thymidine kinase encoded
by this virus (drugs such as acyclovir that are active against herpes simplex
are activated by the viral thymidine kinase). A vaccine is being developed.
|
|
|
Cytomegalovirus
Cytomegalovirus has the largest genome of all herpes viruses and appears only
to replicate in human cells. Its name derives form the fact that, like other
herpes viruses, it can form multinucleated cells (syncytia) with
characteristically staining inclusions. Some cells such as macrophages and
fibroblasts support a productive infection while a latent infection is set up in
several cell types including T lymphocytes and stromal cells of the bone marrow.
There is only one serotype.
Transmission
Cytomegalovirus infection is found in s significant proportion of the
population. As with Epstein-Barr virus (also spread in saliva), seropositivity
increases with age. By college age, about 15% of the US population is infected
and this rises to about half by 35 years of age. The virus is spread in most
secretions, particularly saliva, urine, vaginal secretions and semen (which
shows the highest titer of any body fluid). Cytomegalovirus infection is
therefore sexually transmitted. It can also spread to a fetus in a pregnant
woman and to the newborn via lactation, though there is some doubt about the
importance of milk transmission. In the hospital, the virus can also be spread
via blood transfusions and transplants. In third world countries with more
crowded conditions, the virus is found in a much higher proportion of the
population than in western countries.
Pathogenesis
Cytomegalovirus causes no symptoms in children and at most mild disease in
adults (but see below). The virus first infects the upper respiratory tract and
then local lymphocytes. Circulating lymphocytes then spread the virus to other
lymphocytes and monocytes in spleen and lymph nodes. The virus finally spreads
to a variety of epithelial cells including those of salivary glands, kidney
tubules, testes, epididymis and cervix. Infection is usually asymptomatic
(sub-clinical) but glandular fever is sometimes seen in young adults. The virus
can inhibit T cell responses. The virus elicits both humoral antibodies and
cell-mediated immunity but the infection is not cleared. Cell-mediated immunity,
not humoral antibodies, controls the infection The importance of cell-mediated
immunity stems from the possibility of spread from cell to cell. Although
suppressed, the virus may later reactivate, particularly in cases of
immunosuppression; indeed, infection by the virus can, itself, be
immunosuppressive.
|
| |
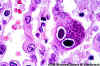 Figure 24A
Figure 24A
H&E stain of lung section showing nuclear inclusions with the appearance of an
"owl's eye". The inclusion is surrounded by a clear
halo that extends to the nuclear membrane.
CMV infection can occur without the typical cytomegalic cells.
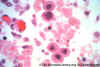 Figure 24B
Figure 24B
H&E stain of CMV-infected cells in lung of AIDS patient. Nuclear
inclusions can be seen
Both images ©
Danny L. Wiedbrauk, William Beaumont Hospital Royal Oak, Michigan
and Joan E.Barenfanger
Memorial Medical Center Springfield, Illinois
and The MicrobeLibrary
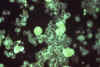 Figure 24C
Figure 24C
Specimen of human embryonic lung
reveals the presence of cytomegalovirus using immunofluorescent technique.
Mag. 25X. CDC/Dr. Craig Lyerla
|
Congenital disease
There are two instances in which cytomegalovirus can cause serious disease.
During a primary infection of the mother, the virus can spread via the placenta
to the fetus and congenital abnormalities can occur; in fact, this virus is the
most common viral cause of congenital disease. Up to one in forty newborns in
the United States are infected by the virus. Abnormalities include microcephaly,
rash, brain calcification and hepatosplenomegaly. These may result in hearing
loss (bilateral or unilateral) and retardation. As might be expected, when
reactivation occurs in a pregnant mother (usually reactivation in the cervix),
the symptoms are less severe because of the mothers seropositivity. In this
case, congenital abnormalities are rare.
Besides infection in utero, infants may be infected perinatally. As
noted above, one tissue in which cytomegalovirus can set up a latent infection
is the cervical epithelium and immunosuppression associated with pregnancy can
lead to reactivation. About 50% of children born to such mothers are infected
and can themselves shed virus within a few weeks. Also breast epithelium can
harbor latent virus that may be similarly reactivated leading to infection of
the infant. In neither case is there usually a problem and the infant remains
asymptomatic.
Neonates may also receive the virus through infected blood transfusions. In
this case, the amount of virus is much higher and symptoms may occur. These
usually consist of pneumonia and hepatitis.
Disease in immunosuppressed patients
In patients who have received an organ transplant or have an
immunosuppressive disease (e.g. AIDS), cytomegalovirus can be a major problem.
Particularly important is cytomegalovirus-retinitis in the eye which occurs in
up to 15% of all AIDS patients. In addition, interstitial pneumonia, colitis,
esophagitis and encephalitis are seen in some patients.
Diagnosis
Most infections are asymptomatic and therefore go undiagnosed. There are
fluorescent antibody (fig 24a) and ELIZA tests. Multinucleated (cytomegalinic) cells with
characteristic inclusions can be seen in biopsies of many tissues (Figure 24).
Treatment
Ganciclovir, which inhibits the replication of all human herpes viruses, is
usually used, especially to treat retinitis. Foscarnet is also approved in the
US. Acyclovir is not effective. A vaccine is being developed but the best way to
avoid the virus is to restrict contact between infected children and pregnant
women. Also since cytomegalovirus is sexually transmitted, condoms can limit
spread.
|
| |
OTHER HERPES VIRUSES
|
| |
Human herpes virus 6
This virus is found worldwide and is found in the saliva of the majority of
adults (>90%). It infects almost all children by the age of two and the
infection is life-long. Again, it replicates in B and T lymphocytes,
megakaryocytes,
glioblastoma cell and in the oropharynx. It can set up a latent infection in T
cells which can later be activated when the cells are stimulated to divide.
Infected cells are larger than normal with inclusions in both cytoplasm and
nucleus. Cell-mediated immunity is essential in control, although infection is
life-long, and the virus can reactivate in immune-suppression. The receptor for
this virus is not known.
Pathogenesis
Human herpes virus-6 has two forms, HHV-6A and HHV-6B. The latter causes exanthem
subitum, otherwise known as roseola infantum. This a common disease of young children (in the US >45% of children
are seropositive for HHV-6 by two years of age) and symptoms include fever and sometimes
upper respiratory tract infection and lymphadenopathy. The symptoms last a few
days after an incubation period of around 14 days. The fever subsides leaving a
macropapular rash on the trunk and neck that last a few days longer. In adults,
primary infection is associated with a mononucleosis. This virus was originally
isolated from patients with a lymphoproliferative disease and may co-infect
HIV-infected T4 lymphocytes exacerbating the replication of HIV. Patients with
HIV have a higher infection rate than the normal population. HHV-6 has
been associated with a number of neurological disorders, including encephalitis and
seizures. It has been postulated to play a role in multiple sclerosis and chronic fatigue immunodeficiency syndrome.
|
|
|
| |
Human herpes virus 7
This virus binds to the CD4 antigen and replicates in T4 (CD4+) cells and is found in the saliva of the
majority of the adult population (>75%). Most people acquire the infection as
children and it remains with them for the rest of their lives. It is similar to
HHV-6 and may be responsible for some cases of exanthem subitum
|
|
|
| |
Human herpes virus 8
This was formerly known as Kaposi's
sarcoma associated herpes virus and is found in the saliva of many AIDS
patients. It infects peripheral blood lymphocytes. The distribution of the virus
may explain why some populations of HIV-infected people go down with Kaposi's
sarcoma while others do not. For further details see the
AIDS/HIV section
|
| |
Herpes B
This is a simian virus found in old world monkeys such as macaques but it can
be a human pathogen in people who handle monkeys (monkey bites are the route of
transmission). In humans, the disease is much more problematic than it is in its
natural host. Indeed, about 75% of human cases result in death with serious
neurological problems (encephalitis) in many survivors. There is also evidence
that the disease can be passed from a monkey-infected human to another human. In
vitro the virus is sensitive to both Acyclovir and Ganciclovir and these are
recommended for therapy. Their efficacy is unknown.
|
|
|
 Return to the Virology section of Microbiology and Immunology On-line
Return to the Virology section of Microbiology and Immunology On-line
 Return to the Home Page of Microbiology and Immunology On-line
Return to the Home Page of Microbiology and Immunology On-line
This page last changed on
Saturday, October 29, 2016
Page maintained by
Richard Hunt
|

 FIGURE 1 Classification of Herpes viruses
FIGURE 1 Classification of Herpes viruses Liquid-Crystalline, Phage-like Packing of Encapsidated DNA in Herpes
Simplex Virus
(F.P.Booy, W.W.Newcomb,
B.L.Trus, J.C.Brown, T.S.Baker, and A.C.Steven, in CELL, Vol 64 pp
1007-1015, March 8, 1991)
Liquid-Crystalline, Phage-like Packing of Encapsidated DNA in Herpes
Simplex Virus
(F.P.Booy, W.W.Newcomb,
B.L.Trus, J.C.Brown, T.S.Baker, and A.C.Steven, in CELL, Vol 64 pp
1007-1015, March 8, 1991)
 Glycoprotein "spikes" on the HSV surface. Glycoprotein B (gB)
is clearly visualised in clusters of spikes about 10 nm in length. Between
the capsid and the envelope is an ill-defined layer of proteins,
collectively known as the tegument.
Copyright
Dr Linda M Stannard,
University of Cape Town, South Africa, 1995 (used with permission)
Glycoprotein "spikes" on the HSV surface. Glycoprotein B (gB)
is clearly visualised in clusters of spikes about 10 nm in length. Between
the capsid and the envelope is an ill-defined layer of proteins,
collectively known as the tegument.
Copyright
Dr Linda M Stannard,
University of Cape Town, South Africa, 1995 (used with permission)
 Figure 7
Figure 7 Figure 9B
Figure 9B Figure 18F
Figure 18F Figure 18I
Figure 18I Figure 23B
Figure 23B















































