|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
|
IMMUNOLOGY - CHAPTER FIFTEEN
MHC: GENETICS AND ROLE IN TRANSPLANTATION
Dr Abdul
Ghaffar
Emertius Professor
Department of Pathology, Microbiology and Immunology
University of South Carolina
|
|
TURKISH |
|
FRANCAIS |
|
SHQIP |
|
PORTUGUES |
|
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|
|

 |
|
Logo image © Jeffrey
Nelson, Rush University, Chicago, Illinois and
The MicrobeLibrary |
|
|
|
|
|
TEACHING
OBJECTIVES
Know
the MHC loci and their products
Understand
the genetic basis of MHC heterogeneity in population
Know
the distribution of MHC molecules on different cells
Know
how MHC antigens are detected (tissue typing)
Understand
the role of MHC in Transplantation, immune functions and disease
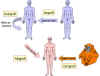 Figure 1 Types of graft
Figure 1 Types of graft |
DEFINITIONS
-
Histocompatibility (transplantation)
antigens
Antigens on tissues and cells
that determine their rejection when grafted between two genetically different
individuals
-
Major histocompatibility (MHC)
antigens
Histocompatibility antigens that cause a very strong immune
response and are most important in rejection
-
MHC complex
Group of genes on
a single chromosome encoding the MHC antigens
-
HLA (human leukocyte antigens)
MHC antigens of man (first detected on leukocytes)
-
H-2 antigens
MHC antigens of
mouse
Types of graft (figure
1)
-
Xenograft
Grafts between
members of different species (also known as heterologous, xenogeneic or
heterografts)
-
Allograft
Grafts between two
members of the same species (also known as allogeneic or homograft)
-
Isograft
Grafts between
members of the same species with identical genetic makeup (identical twins or
inbred animals)
Haplotype
A group of genes on
a single chromosome
|
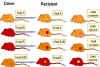 Figure 2 Laws of transplantation
Figure 2 Laws of transplantation
|
PRINCIPLES OF TRANSPLANTATION
(figure 2)
An immunocompetent host recognizes the
foreign antigens on grafted tissues (or cells) and mounts an immune response which
results in rejection. On the other hand, if an immunocompromised host is grafted
with foreign immunocompetent lymphoid cells, the immunoreactive T-cells in the
graft recognize the foreign antigens on the host tissue, leading to damage of
the host tissue.
Host-versus-graft-reaction
The duration of graft survival follows
the order, xeno- < allo- < iso- = auto- graft. The time of rejection also
depends on the antigenic disparity between the donors and recipient. MHC
antigens are the major contributors in rejection, but the minor
histocompatibility antigens also play a role. Rejection due to disparity in
several minor histocompatibility antigens may be as quick or quicker than
rejection mediated by an MHC antigen. As in other immune responses, there is
immunological memory and secondary response in graft rejection. Thus, once a
graft is rejected by a recipient, a second graft from the same donor, or a donor
with the same histocompatibility antigens, will be rejected in a much shorter
time.
|
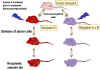 Figure 3 Graft versus host disease
Figure 3 Graft versus host disease |
Graft-versus-host (GVH)
Reaction
Histocompatible lymphoid
cells, when injected into an immunocompromised host, are readily
accepted. However, the immunocompetent T lymphocytes among the grafted
cells recognize the alloantigens and, in response, they proliferate and
progressively cause damage to the host tissues and cells. This condition
is known as graft-versus-host (GVH) disease (figure 3) and is often
fatal.
Common manifestations (figure 4) of GVH reaction are diarrhea, erythema,
weight loss, malaise, fever, joint pains, etc. and ultimately death.
|
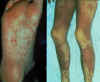 Figure 4 Graft versus host disease
Figure 4 Graft versus host disease |
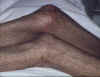 Early, chronic graft-versus-host reaction with widespread, almost confluent hyperpigmented lichenoid papules and toxic epidermal necrosis-like appearance on knee
© Bristol Biomedical Image Archive. Used with
permission
Early, chronic graft-versus-host reaction with widespread, almost confluent hyperpigmented lichenoid papules and toxic epidermal necrosis-like appearance on knee
© Bristol Biomedical Image Archive. Used with
permission
 Late, chronic graft-versus -host reaction with hyperpigmented sclerotic plaques on the back
© Bristol Biomedical Image Archive. Used with
permission
Late, chronic graft-versus -host reaction with hyperpigmented sclerotic plaques on the back
© Bristol Biomedical Image Archive. Used with
permission
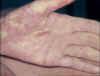 Acute graft-versus-host reaction with vivid palmar erythema ©
Bristol Biomedical Image Archive. Used with permission
Acute graft-versus-host reaction with vivid palmar erythema ©
Bristol Biomedical Image Archive. Used with permission
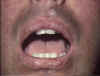 Graft-versus-host reaction with early, chronic, diffuse, widespread lichenoid changes of lips
© Bristol Biomedical Image Archive. Used with
permission
Graft-versus-host reaction with early, chronic, diffuse, widespread lichenoid changes of lips
© Bristol Biomedical Image Archive. Used with
permission
 Graft-versus-host reaction; acute basal cell hydropic degeneration with interepidermal necrotic keratinocytes
Graft-versus-host reaction; acute basal cell hydropic degeneration with interepidermal necrotic keratinocytes
© Bristol Biomedical Image Archive. Used with
permission
 Graft-versus-host reaction; early chronic hyperkeratosis and
hypergranulosis, irregular acanthosis, cytoid body and basal cell hydropic degeneration reminiscent of lichen planus
© Bristol Biomedical Image Archive. Used with
permission
Graft-versus-host reaction; early chronic hyperkeratosis and
hypergranulosis, irregular acanthosis, cytoid body and basal cell hydropic degeneration reminiscent of lichen planus
© Bristol Biomedical Image Archive. Used with
permission
|
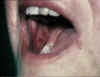 Acute erosions of the buccal mucosa in graft-versus-host reaction
©
Bristol Biomedical Image Archive. Used with permission
Acute erosions of the buccal mucosa in graft-versus-host reaction
©
Bristol Biomedical Image Archive. Used with permission |
 Figure 5 The human MHC gene complex
Figure 5 The human MHC gene complex |
THE MHC GENE COMPLEX
The MHC complex contains a number of
genes that control several antigens, most of which influence allograft
rejection. These antigens (and their genes) can be divided into three major
classes: class I, class II and class III. The
class I and class II antigens are expressed on cells and tissues
whereas as class III antigens are represented on proteins in serum and
other body fluids (e.g.C4, C2, factor B, TNF). Antigens of class III
gene products have no role in graft rejection.
Human MHC
The human MHC is located on chromosome
6.
Class I MHC
The class I gene complex contains
three major loci, B, C and A and other undefined minor loci
(figure 5). Each major locus codes for a polypeptide; the alpha-chain that
contains antigenic determinants, is polymorphic (has many alleles). It
associates with beta-2 microglobulin (beta-chain), encoded by a gene outside the
MHC complex, and expressed on the cell
surface. Without the beta-2 microglobulin, the class I antigen will not be
expressed on the cell surface. Individuals with a defective beta-2
microglobulin gene do not express any class I antigen and hence have a
deficiency of cytotoxic T cells.
Class II MHC
The class II gene complex also
contains at least three loci, DP, DQ and DR; each of these
loci codes for one alpha- and one beta-chain polypeptide which associate together to
form the class II antigens. Like the class I antigens, the
class II antigens are also polymorphic. The DR locus may contain more than one,
possibly four, functional beta-chain genes.
|
 Figure 6A The mouse MHC complex
Figure 6A The mouse MHC complex |
Mouse MHC
The mouse MHC is located on chromosome
17.
Class I MHC
This consists of two major loci,
K
and D. Unlike the human MHC, the mouse class I gene complexes loci are
not together but they are separated by class II and class III genes
(Figure 6A).
Class II MHC
The class II gene complex
contains two loci, A and E, each of which code for one alpha and
one beta chain polypeptide, which form one class II molecule. The mouse
class II gene complex is also known as the I region and the genes in
this complex are referred to as Ir (immune response) genes since they
determine the magnitude of immune responsiveness of different mouse strains to
certain antigens. Products of the A and E loci are also termed IA and IE antigens,
collectively known as Ia antigens.
|
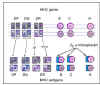 Figure 6B Codominant expression of MHC antigens
Figure 6B Codominant expression of MHC antigens |
MHC ANTIGENS
Nomenclature
HLA specificities are identified by a
letter for locus and a number (A1, B5, etc.) and the haplotypes are
identified by individual specificities (e.g., A1, B7, Cw4, DP5, DQ10, DR8).
Specificities which are defined by genomic analysis (PCR), are names with a
letter for the locus and a four digit number (e.g. A0101, B0701, C0401
etc).
Specificities of mouse MHC (H-2) are
identified by a number. Since laboratory mice are inbred, each strain is
homozygous and has a unique haplotype. The MHC haplotype in these strains is
designated by a 'small' letter (a, b, d, k, q, s, etc.); for example,
the MHC haplotype of Balb/c mice is H2d.
Inheritance
MHC genes are inherited as a group (haplotype),
one from each parent. Thus, a heterozygous human inherits one paternal and one
maternal haplotype, each containing three class-I (B, C and A) and three
class II (DP, DQ and DR) loci. A heterozygous individual will inherit a
maximum of 6 class I specificities (Figure 6). Similarly, the individual
will also inherit DP and DQ genes and express both parental antigens. Since the
class II MHC molecule consists of two chains (alpha and beta), with some
antigenic determinants (specificities) on each chain, and DR alpha- and
beta-chains
can associate in ether cis (both from the same parent) or trans (one
from each parent) combinations, an individual can have additional DR
specificities (Figure 6B). Also, there are more than one functional DR
beta-chain genes (not shown in the figure). Hence, many DR specificities can
be found in any one individual.
|
| |
Crossover
Haplotypes, normally, are inherited
intact and hence antigens encoded by different loci are inherited together
(e.g., A2; B27; Cw2; DPw6; DQw9; DRw2). However, on occasions, there is crossing
over between two parental chromosomes, thereby resulting in new recombinant
haplotypes. Thus, any one specificity encoded by one locus may combine with
specificities from other loci. This results in vast heterogeneity in the MHC
make-up in a given population.
MHC antigen expression on cells
MHC antigens are expressed on the cell
surface in a co-dominant manner: products of both parental genes are
found on the same cells. However, not all cells express both class I and
class II antigens. While class I antigens are expressed on all
nucleated cells and platelets (and red blood cells in the mouse), the expression
of class II antigens is more selective. They are expressed on B lymphocytes, a
proportion of macrophages and monocytes, skin associated (Langerhans) cells,
dendritic cells and occasionally on other cells.
MHC detection by serological test
The MHC class I antigens are
detected by serological assays (Ab and C). Tissue typing sera for the HLA were
obtained, in the past, from multiparous women who were exposed to the
child's paternal antigens during parturition and subsequently developed
antibodies to these antigens. More recently, they are produced by monoclonal
antibody technology. With most laboratories switching to PCR for tissue typing,
the use of serology is rapidly diminishing.
MHC detection by mixed leukocyte
reaction (MLR)
It has been observed that
lymphocytes from one donor, when cultured with lymphocytes from an unrelated
donor, are stimulated to proliferate. It has been established that this
proliferation is primarily due to a disparity in the class II MHC (DR)
antigens and T cells of one individual interact with allogeneic class-II MHC
antigen bearing cells (B cells, dendritic cells, langerhans cells, etc.).
This reactivity was termed mixed leukocyte reaction (MLR) and has been used
for studying the degree of histocompatibility. In this test, the test
lymphocytes (responder cells)are mixed with irradiated or mitomycin C
treated leukocytes from the recipient, containing B-lymphocytes and
monocytes (stimulator cells). The cells are cultured for 4 6 days. The
responder T cells will recognize the foreign class II antigens found on the
donor and undergo transformation (DNA synthesis and enlargement:
blastogenesis) and proliferation (mitogenesis). The T cells that respond to
foreign class II antigens are typically CD4+ TH-1 type cells. These changes
are recorded by the addition of radioactive (tritiated, 3H) thymidine into
the culture and monitoring its incorporation into DNA.
|
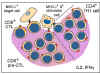 Figure 7 Activation of CTL during MLR
Figure 7 Activation of CTL during MLR |
Generation of cytotoxic T lymphocytes
Another consequence of the MHC antigen
and T cell interaction is the induction of cytotoxic T-lymphocytes. When
T-lymphocytes are cultured in the presence of allogeneic lymphocytes, in
addition to undergoing mitosis (MLR), they also become cytotoxic to cells of the
type that stimulated MLR (figure 7). Thus, T-lymphocytes of 'x' haplotype
cultured over 5 - 7 days with B lymphocytes of 'y' haplotype will undergo mitosis
and the surviving T-lymphocytes become cytotoxic to cells of the 'y' haplotype.
The induction of mitosis in MLR requires disparity of only class II
antigens whereas the induction of cytotoxic T-lymphocytes (CTL) requires
disparity of both class I and class II antigens. However, once
cytotoxic cells have been induced, the effector cytotoxic cells recognize only
class I antigens to cause cytotoxicity.
|
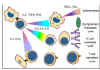 Figure
8 Mechanisms of allograft destruction Figure
8 Mechanisms of allograft destruction |
ALLOGRAFT REJECTION
The clinical significance of the MHC
is realized in organ transplantation. Cells and tissues are routinely
transplanted as a treatment for a number of diseases. However, reaction of the
host against allo-antigens of the graft (HVG) results in its rejection and is
the major obstacle in organ transplantation. The rejection time of a graft may
vary with the antigenic nature of the graft and the immune status of the host
and is determined by the immune mechanisms involved (Figure 8 and Table 1).
Hyper-acute rejection
This occurs in instances when the
recipient has preformed high titer antibodies. A graft may show signs of
rejection within minutes to hours due to immediate reaction of antibodies and
complement.
|
| |
Accelerated (2nd set; secondary)
rejection
Transplantation of a second graft,
which shares a significant number of antigenic determinants with the first one,
results in a rapid (2 - 5 days) rejection. It is due to presence of T-lymphocytes
sensitized during the first graft rejection. Accelerated rejection is mediated by
immediate production of lymphokines, activation of monocytes and macrophages, and
induction of cytotoxic lymphocytes.
|
Table 1.
Different patterns of graft rejection |
|
Type of rejection |
Time taken |
Cause |
|
Hyper-acute
Accelerated
Acute
Chronic |
Minutes-hours
Days
Days - weeks
Months - years |
Preformed anti-donor antibodies
and complement.
Reactivation of sensitized T
cells
Primary activation of T cells
Causes unclear: antibodies,
immune complexes, slow cellular reactions, recurrence of disease. |
Acute (1st set; primary) rejection
The normal reaction that follows the
first grafting of a foreign transplant takes 1 - 3 weeks. This is known as acute
rejection and is mediated by T lymphocytes sensitized to class I and class II
antigens of the allograft, elicitation of lymphokines and activation
of monocytes and macrophages.
|
 Figure
9A Figure
9A
Kidney Transplantation Graft Rejection © Bristol
Biomedical Image Archive. Used with permission |
Chronic rejection
Some grafts may survive for months or
even years, but suddenly exhibit symptoms of rejection. This is referred to as
chronic rejection, the mechanism of which is not entirely clear. The
hypotheses are that this may be due infection, causes which led to failure
of the first organ, loss of tolerance induced by the graft, etc.
Fetus as an Allograft
The fetus in an out-bred mammalian species bears antigens
derived from both the father and the mother. Thus, truly, the fetus is an
allograft and the mother should normally recognize the fetus as foreign and
reject the fetus. Nonetheless, such rejections seldom occur. Thus, mammals
have adapted in a way that allows implantation of their embryos in the
mother's womb and their subsequent survival. There are multiple mechanisms
that play a role, of which the most important being the unique structure and
function of placenta.
Immunologically privileged sites and tissues
There are certain locations in the body in which allografts
are not readily rejected. These include the brain, anterior chamber of the
eye, testis, renal tubule, uterus, etc. This stems from the fact that such
sites may lack of good lymphatic drainage. Also, such tissues may express
molecules such as Fas ligand that kills any immune cell that may come in
contact with these tissues. Additionally, such tissues, may have other
immune suppressor mechanisms. Similarly, there are some tissues that can be
transplanted without matching and without being rejected. Such tissues are
called immunologically privileged tissues. Corneal graft is an excellent
example that enjoys the highest success rate of any form of organ
transplantation. The low incidence of graft rejection is impressive despite
the fact that HLA antigen matching of donor and recipient is not normally
performed. There are many explanations as to why such grafts are accepted.
The avascularity of the graft bed prevents corneal alloantigens from
reaching the regional lymphoid tissues. Also, the corneal antigens may be
masked. Together, such mechanisms fail to activate the immune system of the
recipient.
PROCEDURES TO ENHANCE GRAFT SURVIVAL
In clinical practice, the most
successful transplantation programs have been with kidneys and corneas. However,
other organs are being transplanted with increasing frequency. The success in
these programs has been due to a better understanding of immunological
mechanisms, definition of MHC antigens and development of more effective
immunosuppressive agents.
|
 Figure
9B Figure
9B
Kidney Transplantation Chronic Graft Rejection ©
Bristol Biomedical Image Archive. Used with permission |
Donor selection
Based on extensive experiences with
renal transplants, certain guidelines can be followed in donor selection and
recipient preparation for most organ transplants. The most important in donor
selection is the MHC identity with the recipient; an identical twin is the ideal
donor. Grafts from an HLA-matched sibling have 95-100% chance of success. One
haplotype-identical parent or sibling must match at the HLA D region. A
two
haplotype-distinct donor with a reasonable match for D-region antigen can
also be used. Organs from a two or one DR matched cadaver have been used also
with some success. In every case, an ABO compatibility is essential.
Recipient preparation
The recipient must be infection-free
and must not be hypertensive. One to five transfusions of 100-200 ml whole blood
from the donor at 1-2 week intervals improves the graft survival and is
practiced when possible.
Immunosuppression
Immunosuppressive therapy is most
essential part of allo-transplantation. The most recent and effective family of
agents is cyclosporin A, FK-506 (tacrolimus) and rapamycin. Cyclosporin A and
FK506 inhibit IL-2 synthesis following Ag-receptor binding whereas rapamycin
interferes with signal transduction following IL2 - IL2 receptor interaction. Thus, all
three agents block T cell proliferation in response to antigen. Other
chemical agents used to prevent graft rejection and their modes of action have
been listed in Table 2. Whole body irradiation is used in leukemia patients
before bone marrow transplantation. Antisera against T cells (anti-thymocyte
globulin: ATG) or their surface antigens (CD3, CD4, CD45 on activated T-cells,
CD25:IL-2 receptors) are being used also to achieve immunosuppression (Table 2).
|
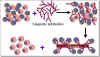 Figure 10 Removal of T cells from marrow graft
Figure 10 Removal of T cells from marrow graft |
Strategies for bone marrow
transplantation
In bone marrow transplantation, the
most crucial factor in donor selection is class II MHC compatibility. Once
again an identical twin is the ideal donor. From poorly matched grafts, T
lymphocytes can be removed using monoclonal antibodies (figure 10). The recipient must be
immunosuppressed. Malignant cells must be eliminated from the recipient blood
(in case of blood-borne malignancies). Methotrexate, cyclosporin and prednisone
are often used to control GVH disease.
|
| |
Other grafts
Corneal grafts do not contain D region
antigens and consequently survival is frequent. Small grafts are better and
corticosteroids are helpful.
Skin allografts have a very poor success
rate and immunosuppressive therapy is relatively ineffective. Nevertheless, they
are often used to provide a temporary covering to promote healing in severe skin
damage. Indeed, there will be no rejection if the host and donor are perfectly
matched (identical twins) or the recipient is tolerant to the donor MHC antigens
(bone marrow chimeras).
|
Table 2.
Examples of selected immunosuppressive agents |
|
agent |
possible mode of action |
application(s) |
|
corticosteroids, prednisone
cyclosporin, FK-506
rapamycin
azathioprine, 6-MP
methotrexate
cyclophosphamide, melphalan |
anti-inflammatory, altering
T-cell and PMN traffic
inhibition of IL-2 synthesis
blocking of IL2-IL2R signal
purine metabolism
folate metabolism
alkylation of DNA, RNA and
proteins |
organ transplant,
hypersensitivity, autoimmune diseases
organ transplant
organ transplant
organ transplant, autoimmuniy
organ transplant, autoimmuniy
organ transplant, autoimmuniy
|
MHC association with diseases
A number of diseases have been found
to occur at a higher frequency in individuals with certain MHC haplotypes. Most
prominent among these are ankylosing spondylitis (B27), celiac disease (DR3) and
Reiter's syndrome (B27). Other diseases associated with different specificities
of the MHC are listed in Table 3. No definite reason is known for this
association. However, several hypotheses have been proposed: antigenic
similarity between pathogens and MHC, antigenic hypo- and hyper-responsiveness
controlled by the class II genes are included among them.
|
Table 3. Examples of significant HLA
and disease associations |
|
Disease |
Associated Alleles |
Frequency in |
Relative Risk |
|
Patients |
Control |
|
Ankylosing spondylitis
|
B27
|
90
|
9
|
87.4
|
| Reiter's disease (syndrome) |
B27 |
79 |
9 |
37.0 |
| Acute anterior uveitis
(figure 11) |
B27 |
52 |
9 |
10.4 |
| Psoriasis vulgaris
(figure 11) |
Cw6 |
87
|
33 |
13.3 |
| Dermatitis herpetiformis
(figure 11) |
DR3 |
85 |
26
|
15.4 |
| Celiac Disease |
DR3 |
79 |
26
|
10.8 |
| Insulin-dependent diabetes
mellitus |
DR3/4 |
91 |
57 |
7.9 |
|
 Figure 11 Psoriasis of the hand
© Bristol Biomedical
Image Archive. Used with permission
Figure 11 Psoriasis of the hand
© Bristol Biomedical
Image Archive. Used with permission |
 Psoriasis © Bristol Biomedical Image Archive.
Used with permission
Psoriasis © Bristol Biomedical Image Archive.
Used with permission
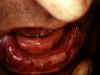 Dermatitis Herpetiformis:
Mouth Mucosa © Bristol Biomedical Image
Archive. Used with permission
Dermatitis Herpetiformis:
Mouth Mucosa © Bristol Biomedical Image
Archive. Used with permission
 Uveitis © Bristol Biomedical Image Archive. Used
with permission
Uveitis © Bristol Biomedical Image Archive. Used
with permission
|
| |
You have learned about
The role of MHC in
host-versus-graft (HGV) and graft-versus-host (GVH) disease.
Genetics of the two MHC
molecules.
The role of polymorphism and
crossover in heterogeneity of MHC antigens in a population.
Methods for detecting MHC antigens
(tissue typing).
Immune mechanisms in transplant
rejection.
Strategies for successful
transplantation.
|
|
|
 Return to the Immunology Section of Microbiology and Immunology
On-line
Return to the Immunology Section of Microbiology and Immunology
On-line
This page last changed on
Thursday, March 31, 2016
Page maintained by
Richard Hunt
|

 Figure 2 Laws of transplantation
Figure 2 Laws of transplantation
 Figure 3 Graft versus host disease
Figure 3 Graft versus host disease Figure 4 Graft versus host disease
Figure 4 Graft versus host disease  Early, chronic graft-versus-host reaction with widespread, almost confluent hyperpigmented lichenoid papules and toxic epidermal necrosis-like appearance on knee
© Bristol Biomedical Image Archive. Used with
permission
Early, chronic graft-versus-host reaction with widespread, almost confluent hyperpigmented lichenoid papules and toxic epidermal necrosis-like appearance on knee
© Bristol Biomedical Image Archive. Used with
permission Acute graft-versus-host reaction with vivid palmar erythema ©
Bristol Biomedical Image Archive. Used with permission
Acute graft-versus-host reaction with vivid palmar erythema ©
Bristol Biomedical Image Archive. Used with permission Graft-versus-host reaction; acute basal cell hydropic degeneration with interepidermal necrotic keratinocytes
Graft-versus-host reaction; acute basal cell hydropic degeneration with interepidermal necrotic keratinocytes
 Acute erosions of the buccal mucosa in graft-versus-host reaction
©
Bristol Biomedical Image Archive. Used with permission
Acute erosions of the buccal mucosa in graft-versus-host reaction
©
Bristol Biomedical Image Archive. Used with permission Figure 5 The human MHC gene complex
Figure 5 The human MHC gene complex Figure 6A The mouse MHC complex
Figure 6A The mouse MHC complex Figure 6B Codominant expression of MHC antigens
Figure 6B Codominant expression of MHC antigens Figure 7 Activation of CTL during MLR
Figure 7 Activation of CTL during MLR Figure
8 Mechanisms of allograft destruction
Figure
8 Mechanisms of allograft destruction Figure
9A
Figure
9A  Figure
9B
Figure
9B Figure 10 Removal of T cells from marrow graft
Figure 10 Removal of T cells from marrow graft Figure 11 Psoriasis of the hand
© Bristol Biomedical
Image Archive. Used with permission
Figure 11 Psoriasis of the hand
© Bristol Biomedical
Image Archive. Used with permission Psoriasis © Bristol Biomedical Image Archive.
Used with permission
Psoriasis © Bristol Biomedical Image Archive.
Used with permission Uveitis © Bristol Biomedical Image Archive. Used
with permission
Uveitis © Bristol Biomedical Image Archive. Used
with permission




