| x | x | |||||||||||||||||||||||||
 |
||||||||||||||||||||||||||
| BACTERIOLOGY | IMMUNOLOGY | MYCOLOGY | PARASITOLOGY | VIROLOGY | ||||||||||||||||||||||
Dr Richard Hunt |
||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||
|
Let us know what you think |
||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||
|
TEACHING
OBJECTIVES To learn how cells become transformed by the virus To learn the differences between DNA and RNA tumor viruses To
understand how RNA viral oncogenes result in cell transformation |

There are two classes of these genes in which altered expression can lead to loss of growth control:
Viruses are involved in cancers because they can
either carry a copy of one of these genes or can alter expression of the cell's
copy of one of these genes. These are the oncogenic virus (otherwise known as
oncoviruses or tumor viruses). |
|||||||||||||||||||||||||
|
To understand the discovery of cellular proto-oncogenes To learn how cellular oncogenes may cause cancer in the absence of a virus To learn understand how these discoveries led to the discovery of anti-oncogenes To understand how the discovery of anti-oncogenes showed how DNA viruses may cause cancer |
CLASSES OF TUMOR VIRUSES There are two classes of tumor viruses:
We shall see that these two classes have very different ways of reproducing themselves but they often have one aspect of their life cycle in common: the ability to integrate their own genome into that of the host cell. Such integration is not, however, a pre-requisite for tumor formation.
TRANSFORMATION AND ONCOGENES If a virus takes up residence in a cell and alters the properties of that cell, the cell is said to be transformed. Transformation by a virus is the change in the biological properties of a cell that results from the regulation of the cell by viral genes and that confer on the infected cells certain properties of neoplasia. Transformation often includes loss of growth control, anchorage-independent growth, ability to invade extracellular matrix, dedifferentiation and immortalization. In carcinomas, many epithelial cells undergo an epithelial-mesenchymal transformation. Transformed cells often exhibit chromosomal aberrations and the changes seen in transformation often, but not always, result from the integration of the viral genome into the host cell's chromosomes. The region of the viral genome (DNA in DNA tumor-viruses or RNA in RNA-tumor viruses) that can cause a tumor is called an oncogene. This foreign gene can be carried into a cell by the virus and cause the host cell to take on new properties. The discovery of viral oncogenes in retroviruses led to the finding that they are not unique to viruses and homologous genes (called proto-oncogenes) are found in all cells. Indeed, it is likely that the virus picked up a cellular gene during its evolution and this gene has subsequently become altered. Normally, the cellular proto-oncogenes are not expressed in a quiescent cell since they are involved in growth (which is not occurring in most cells of the body) and development; or they are expressed under strict control by the cell. However, they may become aberrantly expressed when the cell is infected by tumor viruses that do not themselves carry a viral oncogene. We shall see later how this happens but it is clear that a virus may cause cancer in two ways: It may carry an oncogene into a cell or it may activate a cellular proto-oncogene. The discovery of cellular oncogenes opened the way to the elucidation of mechanisms by which non-virally induced cancers may be caused. We shall investigate what the protein products of the viral and cellular oncogenes do in the infected cell and in cells in which cellular proto-oncogenes are expressed. We shall see that their functions strongly suggest mechanisms by which cells may be transformed to a neoplastic phenotype. The discovery of cellular oncogenes led to the discovery of another class of cellular genes, the tumor repressor (suppressor) genes or anti-oncogenes. Initially, the involvement of viral and cellular oncogenes in tumors caused by retroviruses was much more apparent than the involvement of the DNA tumor virus oncogenes but the discovery of tumor repressor genes (as a result of our knowledge of how retroviruses cause cancer) led to the elucidation of the mode of action of DNA virus oncogenes. It should be noted that while retroviruses have been instrumental in elucidation of the mechanisms of oncogenesis, most human cancers are probably not the result of a retroviral infection although retroviruses are important in cancers in some animals. It is becoming much more apparent that many human tumors may result from infection by DNA tumor viruses. |
|||||||||||||||||||||||||
 The information flow in DNA tumor viruses is similar to that in eucaryotic
cells
The information flow in DNA tumor viruses is similar to that in eucaryotic
cellsFigure 1 |
||||||||||||||||||||||||||
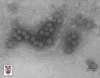 Papilloma virus Copyright 1994 Veterinary Sciences
Division, Queens University Belfast
Papilloma virus Copyright 1994 Veterinary Sciences
Division, Queens University Belfast |
DNA TUMOR VIRUSES DNA tumor virus have a DNA genome that is transcribed into RNA which is translated into protein (figure 1). They have two life-styles:
|
|||||||||||||||||||||||||
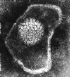
 Papilloma virus Copyright Dr
Linda M Stannard, 1995
(used with permission)
Papilloma virus Copyright Dr
Linda M Stannard, 1995
(used with permission)
Figure 2
|
DNA TUMOR VIRUSES INVOLVED IN HUMAN CANCERS The first DNA tumor viruses to be discovered were rabbit fibroma virus and Shope papilloma virus, both discovered by Richard Shope in the 1930s. Papillomas are benign growths, such as warts, of epithelial cells. They were discovered by making a filtered extract of a tumor from a wild rabbit and injecting the filtrate into another rabbit in which a benign papilloma grew. However, when the filtrate was injected into a domestic rabbit, the result was a carcinoma, that is a malignant growth. A seminal observation was that it was no longer possible to isolate infectious virus from the malignant growth. This was because the virus had become integrated into the chromosomes of the malignant cells.
SMALL DNA TUMOR VIRUSES
FAMILY: PAPILLOMAVIRIDAE
|
|||||||||||||||||||||||||
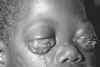
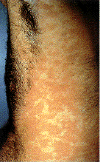 Epidermodysplasia verruciformis. This widespread, markedly
pruritic, erythematous eruption was eventually found to be caused by human papillomavirus infection.
International Association of Physicians in AIDS Care
Epidermodysplasia verruciformis. This widespread, markedly
pruritic, erythematous eruption was eventually found to be caused by human papillomavirus infection.
International Association of Physicians in AIDS Care
|
FAMILY: POLYOMAVIRIDAE
|
|||||||||||||||||||||||||
|
WEB RESOURCES
Epidermodysplasia
verruciformis |
||||||||||||||||||||||||||
|
|
FAMILY: ADENOVIRIDAE
|
|||||||||||||||||||||||||
|
Tumor antigens are oncogenes Tumors caused by papilloma virus, adenovirus or polyoma virus contain viral DNA but do not produce infectious virus. The presence of the virus, however, elicits the formation of antibodies against the tumor antigens. In the case of adenoviruses, only part of the viral genome is found in the host cell chromosomes whereas SV40 may integrate part or all of its genome. Whether or not the whole SV40 genome is integrated, only a part of the genome is transcribed into mRNA and protein and this is the region that encodes the early functions of the virus replication cycle. Many DNA viruses have early and late functions. Early functions are the result of the expression of proteins that prime the cell for virus production and are involved in viral DNA replication. These proteins are expressed before genome replication and do not usually end up in the mature virus particle. Late functions are the results of the expression of viral structural proteins that combine to form the mature virus. They are expressed during and after the process of DNA replication. Since early functions are involved in the replication of the viral genome, it is not surprising that they can also alter the replication of host cell DNA. SV40 expresses two such proteins, the T antigens (large T and small T antigen). The large T antigen acts as a cis-regulatory element at the level of viral DNA replication by binding to the origin of replication and stimulating transcription. It can also bind to and modulate the activity of host cell DNA polymerase alpha. As we shall see later, DNA replication in the cell is controlled by suppressor proteins (the best studied of which are the retinoblastoma (Rb) and p53 suppressor proteins). SV40 large T antigen can bind directly to these proteins and inactivate them, thereby inducing the cell to go from Go to S phase. Because polyoma viruses have a small genome, they rely on many cell functions for DNA replication and it is important that the virus causes the cell to enter S phase because it creates a suitable environment for viral DNA replication.
A second T antigen (small T antigen) interacts with a family of cellular phosphatases (called pp2A) which results in the failure of certain cellular proteins to be phosphorylated, thereby relieving cell cycle arrest. In mouse polyoma virus, there is a middle T antigen which can also act as an oncogene. Similarly, in adenovirus-induced tumors, only a part of the viral genome becomes integrated and again it is the early region genes. This region codes for the E1A and E1B proteins. In papilloma virus-induced tumor, again, two early genes, E6 and E7, are expressed. Thus, papilloma, polyoma and adenoviruses seem to cause cell transformation in a similar manner: the integration of early function genes into the chromosome and the expression of these DNA synthesis-controlling genes without the production of viral structural proteins. As we shall see later, all three virus types induce cell proliferation by interacting with tumor suppressor genes. Two important points that should be emphasized about T antigens of DNA tumor viruses as oncogenes:
These properties should be contrasted with retroviral oncogenes to be discussed later
|
||||||||||||||||||||||||||
 Herpes virus. Negative stain Copyright Dr
Linda M Stannard,
University of Cape Town, South Africa, 1995 (used
with permsssion).
Herpes virus. Negative stain Copyright Dr
Linda M Stannard,
University of Cape Town, South Africa, 1995 (used
with permsssion).  Liquid-Crystalline, Phage-like Packing of Encapsidated DNA in Herpes Simplex Virus
(F.P.Booy, W.W.Newcomb, B.L.Trus, J.C.Brown, T.S.Baker,
and A.C.Steven, in CELL, Vol 64 pp 1007-1015, March 8, 1991)
Liquid-Crystalline, Phage-like Packing of Encapsidated DNA in Herpes Simplex Virus
(F.P.Booy, W.W.Newcomb, B.L.Trus, J.C.Brown, T.S.Baker,
and A.C.Steven, in CELL, Vol 64 pp 1007-1015, March 8, 1991) Herpes Simplex Virus (TEM x169,920)
©
Dennis Kunkel Microscopy, Inc.
Used with permission
Herpes Simplex Virus (TEM x169,920)
©
Dennis Kunkel Microscopy, Inc.
Used with permission
Figure 6 |
COMPLEX TUMOR VIRUSES FAMILY: HERPESVIRIDAE
|
|||||||||||||||||||||||||
 Figure 7A Epstein- Barr Virus |
|
|||||||||||||||||||||||||
 Figure 7E
Figure 7EEarly oral hairy leukoplakia (OHL) on the lateral border of the tongue. HIV reduces immunologic activity, the intraoral environment is a prime target for chronic secondary infections and inflammatory processes, including oral hairy leukoplakia, which is due to the Epstein-Barr virus under immunosuppressed conditions CDC
|
Human Herpes Virus 8 (HHV-8, Kaposi's Sarcoma Herpes Virus) HHV-8 infects lymphocytes and epithelial/endothelial cells and is the causative agent of Kaposi's sarcoma. It has also been associated with hematologic malignancies, including primary effusion lymphoma, multicentric Castleman's (also Castelman's) disease (MCD), MCD-related immunoblastic/plasmablastic lymphoma and various atypical lymphoproliferative disorders. EBV and HHV-8 have been found to be associated with oral lesions and neoplasms in HIV-infected patients. Among these diseases is oral hairy leukoplakia (OHL, figure 7E) which is benign and causes white thickenings on the tongue epithelium in which these viruses proliferate. This herpes virus (figure 7F) is frequently associated with Kaposi's sarcoma but this disease is now thought probably to be caused by human herpes virus 8. For more on herpes viruses and the diseases that
they cause, go to Virology Chapter 11
Herpes
Viruses
|
|||||||||||||||||||||||||
 This woman has hepatitis B and is suffering from liver cancer. She was a Cambodian refugee
and died 4 months after she arrived in a refugee camp (average life expectancy after diagnosis of liver cancer is 6 months)
Immunization Action Coalition Courtesy of Patricia Walker, MD, Ramsey Clinic Associates, St. Paul, MN
This woman has hepatitis B and is suffering from liver cancer. She was a Cambodian refugee
and died 4 months after she arrived in a refugee camp (average life expectancy after diagnosis of liver cancer is 6 months)
Immunization Action Coalition Courtesy of Patricia Walker, MD, Ramsey Clinic Associates, St. Paul, MNFigure 8 |
FAMILY: HEPADNAVIRIDAE
|
|||||||||||||||||||||||||
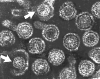 Hepatitis B virions: two exposed cores (indicated by
arrows)
Hepatitis B virions: two exposed cores (indicated by
arrows) |
|
|||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||
|
|
RNA tumor viruses are different from DNA tumor viruses in that their genome is RNA but they are similar to many DNA tumor viruses in that the genome is integrated into the host genome. Since RNA makes up the genome of the mature virus particle, it must be copied to DNA prior to integration into the host cell chromosome. This life style (figure 10) goes against the central dogma of molecular biology in which that DNA is copied into RNA. RNA tumor viruses or oncornaviruses are members of the retrovirus family.
Retrovirus structure The outer envelope comes from the host cell plasma membrane (figure 11). Coat proteins (surface antigens) are encoded by env (envelope) gene and are glycosylated. One primary gene product is made but this is cleaved so that there are more than one surface glycoprotein in the mature virus (cleavage is by host enzyme in the Golgi apparatus). The primary protein (before cleavage) is made on ribosomes attached to the endoplasmic reticulum and is a transmembrane (type 1) protein. Inside the membrane is an icosahedral capsid containing proteins encoded by the gag gene (Group- specific AntiGen). Gag- encoded proteins also coat the genomic RNA. Again there is one primary gene product. This is cleaved by a virally-encoded protease (from the pol gene) There are two molecules of genomic RNA per virus particle with a 5' cap and a 3' poly A sequence. Thus, the virus is diploid. The RNA is plus sense (same sense as mRNA). About 10 copies of reverse transcriptase are present within the mature virus, these are encoded by the pol gene. Pol gene codes for several functions (again, as with gag and env, a polyprotein is made that is then cut up)
|
|||||||||||||||||||||||||
 Structure of RSV protease bound to a peptide analog of the HIV cleavage
site
Structure of RSV protease bound to a peptide analog of the HIV cleavage
siteRequires Chime plug-in. Figure 12 |
The pol gene products are:
|
|||||||||||||||||||||||||
 Human T lymphocyte with HTLV-1 infection
(RNA virus, Retroviridae Family). The virus is in a large clump in the
corner.
©
Dennis Kunkel Microscopy, Inc.
Used with permission
Human T lymphocyte with HTLV-1 infection
(RNA virus, Retroviridae Family). The virus is in a large clump in the
corner.
©
Dennis Kunkel Microscopy, Inc.
Used with permission
Figure 13 |
GROUPS OF RETROVIRUSES ONCOVIRINAE Viruses in this group that cause tumors in humans are: HTLV-1 (human T-cell lymphotropic virus-1, figure 13) which causes Adult T-cell Leukemia (Sezary T-cell Leukemia). This disease is found in some Japanese islands, the Caribbean, Latin America and Africa. HTLV-1 is sexually transmitted
HTLV-2 (human T-cell lymphotropic virus-2) which causes Hairy Cell Leukemia. The virus is endemic to very specific regions of the Americas, particularly in native American populations.
LENTIVIRINAE
SPUMAVIRINAE
|
|||||||||||||||||||||||||
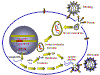 Stages in the productive infection of a cell by a retrovirus
Stages in the productive infection of a cell by a retrovirusFigure 14 |
INFECTION AND TRANSFORMATION OF A CELL BY A RETROVIRUS
The following stages occur in the infection process (figure 14): 1) Binding to a specific cell surface receptor 2) Uptake by endocytosis or by direct fusion to the plasma membrane. The virus may require entry into a low pH endosome before fusion can occur, although some (e.g. HIV) can fuse directly with the plasma membrane 3) RNA (plus sense) is copied by reverse transcriptase to minus sense DNA. Here, the polymerase is acting as an RNA-dependent DNA polymerase. Since reverse transcriptase is a DNA polymerase, it needs a primer. This is a tRNA that is incorporated into the virus particle from the previous host cell. 4) RNA is displaced and degraded by a virus-encoded RNase H activity. Reverse transcriptase now acts as a DNA-dependent DNA polymerase and copies the new DNA into a double strand DNA. This DNA form of the virus is known as a the provirus. 5) Double strand DNA is circularized and integrated into host cell DNA (see below) using a virally encoded integrase enzyme. This DNA is copied every time cellular DNA is copied. Thus, at this stage the provirus is just like a normal cellular gene. 6) Full length, genomic RNA (plus sense) is copied from the integrated DNA by host RNA polymerase II which normally copies a gene to mRNA. The genomic RNA is capped and poly adenylated, just as an mRNA would be.
Note that mRNA comes from splicing genomic RNA or is the genomic RNA. As a result, both mRNA and genomic RNA must be the same sense - since mRNA must be plus sense, the genomic RNA of all retroviruses must also be plus sense. An advantage of this mode of replication is that it allows growth in terminally differentiated cells since the only host cell polymerase usurped by the virus is RNA polymerase II which is present in all cells.
|
|||||||||||||||||||||||||
|
MECHANISM OF VIRAL GENOME REPLICATION If host RNA polymerase II is used to copy the DNA back to RNA, there are major problems with having a DNA provirus form but an RNA genome in the mature virus particle These problems include:
Failure of RNA polymerase II to copy the entire gene This means that either the DNA copy of the viral RNA genome virus must integrate
into host DNA downstream from a host promotor and upstream from host termination sites
(a tall order indeed!) or it must find a way of providing its own control
sequences (which, as we said, are not copied into progeny genome). It does the latter in a
most complex manner. |
||||||||||||||||||||||||||
|
A
B Figure 15 |
How can a retrovirus provide its own control promotors and enhancers if they are not
transcribed when the DNA provirus is copied to the genomic RNA form?
Here is a brief (and very incomplete) summary of how a retrovirus does it: 1) The viral RNA is composed of three regions. At each end are repeats (called, not
surprisingly, terminal repeats). The repeat sequences (R) (shown in green in
figure 15) do not
code for proteins. In between the two repeats, there is a unique (not repeated) region
that contains the viral genes that code for the proteins (GAG, POL and ENV) plus
other unique sequences at either end that do not code for protein. Near the 5' end
of the RNA genome is the U5 region and near the 3' end is the U3 region. PBS (in
figure 15) is the primer binding site. The tRNA binds here when
reverse transcriptase
starts copying the RNA. PPT is a polypurine tract. |
|||||||||||||||||||||||||
|
MOVIE LTR formation |
2) In the integrated form (when transcribed into DNA and inserted into the host cell chromosome), the provirus is more complicated. We find that part of the 3' unique region (called U3) of the RNA genome has been copied and transposed to the opposite end of the genome. Conversely, part of the 5' end of the unique region (called U5) has been copied and transposed to the other end. This gives the integrated DNA the structure shown in figure 15B. For convenience, only one strand of the DNA is shown. Now, of course, there are larger
terminal repeats since the U3 and U5 regions are also repeated. The U3-R-U5 regions
are known as long terminal repeats or LTRs. The U3 region contains all of
the promotor information that is necessary to start RNA transcription at the beginning
of the R (repeat) region while the U5 region contains all of the information necessary
to terminate after the other R repeat. In addition, the LTRs contain information that
enhances the degree of transcription of the three retroviral genes (enhancer regions).
These enhancers can be up or downstream from the protein-encoding part of
the genes. |
|||||||||||||||||||||||||
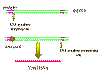 The transcription of a retrorviral DNA with LTRs by RNA
polymerase II results in the loss of the LTRs
The transcription of a retrorviral DNA with LTRs by RNA
polymerase II results in the loss of the LTRs Figure 16 Animated version here |
Host RNA polymerase II copies the provirus DNA to genomic RNA which can be also spliced to mRNAs. Since the polymerase starts after the promotor (in U3), at the transcription initiation site, it begins exactly at the beginning of the R region (figure 16). Thus we get a faithful (almost - see below) copy of the RNA that entered the cell. The termination sequences and poly A signal are in U5 which is also not copied. Because of this mechanism, there can be only one promotor site (from U3) for all three viral genes so they must be all transcribed together. Splicing enzymes, from the host cell nuclear splicing machinery, cut the primary transcript to form the individual mRNAs where necessary. (See chapter 7, HIV in which this has been well elucidated). Unlike the situation with DNA tumor viruses, there is no distinction between early/late functions. You may ask why, if U5 contains the termination and polyadenylation sites, does the transcript not just terminate at the end of the first R region of the LTR (figure 15b) and never get into the structural genes. The termination site in the first U5 is suppressed, often by complex secondary structure mechanism. In some retroviruses there is a sequence in the gag gene that provides the context to suppress the termination activity of the first U5. Clearly the second U5 does not have a gag gene following it. This strategy of virus replication in which viral RNA is first copied to DNA (by reverse transcriptase) which then gives rise to mRNA and protein poses another problem for the virus. The initial step (RNA to DNA) is carried out by a viral enzyme which is not normally in the cell. Yet this transcription step must occur before any mRNA transcription or protein translation can occur. The problem is solved by the virus carrying about 10 copies of the reverse transcriptase protein into the cell with it. These were packaged when the virus was assembled in the previous host cell. In theory, the viral genomic RNA coming into the cell could act as an mRNA but it is too coated with protein to do so. Thus, new mRNA must be made which requires the synthesis of reverse transcriptase.
|
|||||||||||||||||||||||||
|
ONCOGENES IN RETROVIRUSES |
||||||||||||||||||||||||||
 Typical retrovirus structure and the structure of a retrovirus with an
oncogene (Rous Sarcoma Virus)
Typical retrovirus structure and the structure of a retrovirus with an
oncogene (Rous Sarcoma Virus)
Figure 17
|
The structure shown in figure 15A and the upper part of figure 17 is that of a typical retrovirus with three structural genes (gag, pol and env) but none of these is oncogenic. If the virus is to transform a cell, it must have sequences that alter cellular DNA synthesis and provide the other functions that are typical of a transformed cell. These are in addition to the gag/pol/env genome. Thus we also find an ONCOGENE (onc) in the viral genome of many retroviruses that transform cells to neoplasia (figure 17). It should be emphasized that the oncogene in RNA tumor viruses is not necessary for viral replication. It is an additional gene that gives the virus its capacity to transform the host cell.
Definition of virally-induced transformation: The changes in the biologic function
and antigenic specificity of a cell that result from integration of viral genetic
sequences into the cellular genome and that confer on the infected cell certain properties
of neoplasia. Note, however, that transformation can be induced by factors other than
viruses e.g. carcinogens. What are the oncogenic genes in retroviruses? In retroviruses, these were first discovered as an extra gene in Rous sarcoma virus (RSV) (figure 17). This gene was called src (for sarcoma). src is not needed for viral replication. It is an extra gene to those (gag/pol/env) necessary for the continued reproduction of the virus. RSV has a complete gag/pol/env genome. Deletions/mutations in src abolish transformation and tumor promotion but the virus is still capable of other functions. RSV is unusual in that it has managed to retain its whole genome of gag/pol/env.
|
|||||||||||||||||||||||||
|
In sharp contrast to RSV, many retroviruses have lost part of their genome to accommodate an oncogene (figure 18). This has two consequences: 1) The protein encoded by the oncogene is often part of a fusion protein with other virally-encoded amino acids attached 2) Virus is in trouble as it cannot make all of itself. To replicate and bud from the host cell needs products of another virus, that is a helper virus. About forty oncogenes have now been identified. Note that they are referred so by a three letter code (e.g. src, myc) often reflecting the virus from which they were first isolated. Some viruses can have more than one oncogene (e.g. erbA, erbB). Because they are viral oncogenes, we put a letter v in front of the three letter name. Here are a few of the most studied:
|
||||||||||||||||||||||||||
|
CELLS HAVE PROTO-ONCOGENES Once retroviral oncogenes had been discovered, a surprising observation was made: Unlike the situation with DNA virus oncogenes which are true viral genes, there are homologs of all retrovirus oncogenes in cells that are not infected by a retrovirus. These cellular homologs are often genes involved in growth control and development/differentiation (as might be expected) and have important non-transforming functions in the cell; some can cause cancer under certain circumstances and, presumably, those not shown to cause cancer have the ability to do so under the correct conditions. The cellular homologs of viral oncogenes are called proto-oncogenes. To distinguish viral oncogenes from cellular proto-oncogenes, they are often referred to as v-onc and c-onc respectively. Note that c-oncs are not identical to their corresponding v-oncs. It appears that the virus has picked up a cellular growth controlling or differentiation gene and, after the gene was acquired by the virus, it has been subject to mutation. Definition of a proto-oncogene: A host gene that is homologous to an
oncogene that is
found in a virus but which can induce transformation only after being altered (such as
mutation or a change of context such as coming under the control of a highly active
promotor). It usually encodes a protein that functions in DNA replication or growth
control at some stage of the normal development of the organism. Characteristics of cellular proto-oncogenes 1) These are typical cellular genes with typical control sequences. As with most eucaryotic genes, most have introns (while retroviral oncogenes - v-oncs - do not) 2) They show normal Mendelian inheritance because they are normal genes, essential to the functions of the cell. 3) As with all genes in the eukaryotic genome, they are always at same place in genome (cf. what would be expected of endogenous retroviruses that had, over time, become incorporated into the cellular genome) 4) There are no LTR sequences (v-oncs always are in an LTR context) 5) Viral oncogenes are most like the c-onc of the animal from which the virus is thought to have acquired the gene. Thus, v-src of RSV is more like chicken src than human src. Note that v-onc was long ago acquired accidentally by the virus from the genome of a previous host cell 6) Cellular oncogenes are expressed by the cell at some period in the life of the cell, often when the cell is growing, replicating and differentiating normally. They are usually proteins that are involved in growth control. 7) Cellular oncogenes are highly conserved If v-onc and c-onc are so alike, why does the v-onc introduced by a virus cause havoc in the cell? This is due to differences in the genes, mutations that have occurred in the gene once it was picked up by the virus. Such changes include:
|
||||||||||||||||||||||||||
 Some retroviruses that have an oncogene that
replaces their normal genes
Some retroviruses that have an oncogene that
replaces their normal genesFigure 18 |
Chronically transforming retroviruses do not have a v-onc The observation that an acutely transforming virus such as RSV contains an extra gene, the oncogene, explains their high neoplastic potential but, in contrast, chronically-transforming retroviruses only produce tumors slowly and they carry no gene equivalent to a v-onc. At best, these viruses have just the three usual viral genes (gag/pol/env). An example is avian leukosis virus (ALV) (figure 18). How do chronically transforming viruses induce a tumor if they do not have an oncogene? A seminal observation was made: Just as any other retrovirus does, ALV can integrate
into the cell genome at many different sites but, in ALV-induced tumors, the virus
is ALWAYS found in a similar position (very important!). This means that the
crucial transforming event must be rare and that the cells that form the tumor are a clone
(cf. the acute transformers which are found all over the place). In all cases of ALV-induced tumors, the viral genome is inserted near a cellular gene called c-myc.
This is the cellular proto-oncogene that, in an altered form (i.e. as a
v-onc), is
carried by some acutely transforming retroviruses (e.g. avian myelocytoma virus
which causes carcinoma, sarcomas and leukemias). In addition, the level of translation of
c-myc in the ALV-transformed cell is much greater than in uninfected cells. Thus,
inserting the genome of ALV or other chronically transforming retroviruses next to a
c-onc has the same effect as carrying in a v-onc. |
|||||||||||||||||||||||||
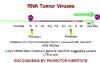 Oncogenesis by
promotor insertion
Oncogenesis by
promotor insertion Figure 19 |
So, during integration, the virus comes to lie upstream from c-myc which then comes under the influence of the strong LTR promotors of the virus which leads to over expression of c-myc. This is called oncogenesis by promotor insertion (Figure 19). But in some tumors the virus is downstream from the c-myc gene. However, we saw that LTRs also have enhancers in addition to promotors. We know that enhancer sequences can be upstream or downstream to have their effect. This is called oncogenesis by enhancer insertion (Figure 20). Why is insertion near c-myc important? The protein coded for by this gene is found in the nucleus of normal cells and is involved in control of DNA synthesis. It can be shown that over-expression of c-myc leads to rapid DNA replication.
|
|||||||||||||||||||||||||
|
A
B
|
Can cellular ongogenes be involved in non-virally
induced cancer?
Once it had been shown that viruses can either bring an oncogene into the cell or can take control of a cellular proto-oncogene to give rise to a tumor, the question arose of whether cellular proto-oncogenes could give rise to tumors in the absence of retroviral infection. The answer is yes! Other chromosomal rearrangements can bring a c-onc under the control of the wrong promotor/enhancer (Figure 21). Alternatively, the c-onc might be mutated in a particular way so that it was over-expressed or it might code for a mutant protein with an altered function. Chromosomal mapping allows the precise localization of the site of a gene on a particular chromosome and many cancers are associated with alterations in chromosomes, particularly translocations (the breakage of a chromosome so that the two parts associate with two parts of another chromosome). Many break sites in tumor cells are very close to a known c-onc. This is highly suggestive and unlikely to have occurred by chance!
* In Burkitt's lymphoma the c-myc on chromosome 8 is brought to a site on chromosome 14 close to the gene for immunoglobulin heavy chains. It seems that the proto-oncogene may thus be brought under the control of the immunoglobulin promotor, which is presumably very active in B lymphocytes. This explains why this tumor arises in B cells. In other lymphomas, a c-onc is brought next to the immunoglobulin light chain promotor. These are also B cell lymphomas. Epstein-Barr virus is probably the cause of Burkitt's lymphoma. This is a herpes virus and herpes viruses commonly cause chromosomal breaks. If such a break causes an 8:14 translocation, the cell's myc gene will become adjacent to the cell's immunoglobulin promotor and c-myc expression will rise in the cells in which this promotor is active.
|
|||||||||||||||||||||||||
|
Is there evidence that mutations in cellular oncogenes might also result in transformation? The best evidence comes from the cellular oncogene that is the homologue of the viral oncogene found in the Harvey strain of murine sarcoma virus (the v-onc is called HaRas). This c-onc was isolated from bladder carcinomas and compared to the normal c-onc proto-oncogene. In many tumor cells only one change was found in the amino acid sequence of the protein; glycine at amino acid position 12 was changed to valine. At position 12 only glycine and proline gave normal growth. All other amino acids at this position gave a transformed cell. In a lung carcinoma, the transforming DNA also contained c-HaRas, again it had a point mutation, this time at position 61.
|
||||||||||||||||||||||||||
|
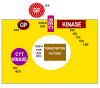
What is the normal function of oncogenes? As mentioned above, c-oncs are normal cellular genes that are expressed and function at some stage of the life of the cell. We should expect them to be involved in DNA synthesis or perhaps the signaling pathways that lead to proliferation. More than 40 oncogenes have been identified and there are probably a few undiscovered ones. We can sub-divide the cellular oncogenes into those that encode nuclear proteins and those that encode extra-nuclear proteins. The latter are mostly associated with the plasma membrane of the cell (Figure 22 and 23). : e.g. myc, myb. These are involved in control of gene expression (that is the regulation of transcription - they are transcription factors) or the control of DNA replication. Neoplasia is associated with elevated transcription of the oncogene but strong expression is not always necessary, rather there is a need to make the gene constitutively active rather than under control of normal regulatory processes. Products of oncogenes that are cytoplasmic or membrane-associated proteins: e.g. abl, src, ras. This type does not exhibit altered expression but seems to convert from proto-oncogene to oncogene by mutation. Thus, in src-induced tumors, strong over expression of the oncogene has no effect.
|
||||||||||||||||||||||||||
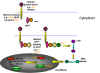 Ways in which altered proto- oncogenes might lead to cell
transformation
Ways in which altered proto- oncogenes might lead to cell
transformation Figure 22
|
In each of these cases, the mutation is dominant. Thus, for example if one allele of erb-B (a homolog of the EGF receptor) is mutated so that it is a constitutively switched on (i.e. does not need epidermal growth factor to bind to switch on the tyrosine kinase activity), then the signal is on, regardless of the fact that the other allele is normal. |
|||||||||||||||||||||||||
 Dominant mutations are function
gained
Dominant mutations are function
gained Figure 24 |
ANTI-ONCOGENES (Tumor suppressor genes) The way in which retroviruses cause tumor formation via oncogenes was established before anything was known about how DNA tumor viruses cause tumors. Certainly, DNA tumor viruses carry oncogenes (e.g. SV40 T-antigen) but how do these proteins, encoded in true viral genes with no cellular homologs, cause the formation of tumors? It has long been known that most tumors are the result of dominant mutations, i.e. a function is gained that makes the cell grow when it should not (Figure 24). For example, as noted above, if we have a receptor that sends a signal when it binds a growth factor by switching on its tyrosine kinase activity and that receptor becomes mutated so that its tyrosine kinase activity is permanently activated, the cell will get the aberrant growth signal even in the heterozygote. Thus the mutant allele is dominant over the normal allele.
|
|||||||||||||||||||||||||
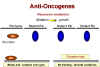 Recessive mutations are functions lost
Recessive mutations are functions lost Figure 24 |
Retinoblastoma: A recessive tumor There is a curious class of tumors that do not fit the usual characteristics in which the mutant oncogene is dominant over the wild type. In retinoblastoma, there appears to be a lesion that is recessive, that is the cancer causing mutation causes a loss of function (Figure 24). (This is recessive because, in a diploid organism, there are two genes. If one allele is mutated so that it does not work, the other can still code for the normal protein and function is retained. In order to lose the function and have no protein being made, both genes must be mutated, i.e. we have a recessive mutation). Thus it appears that the protein that is encoded in the retinoblastoma (Rb) gene is a growth suppressor. If a homozygous mutation occurs in the Rb gene, there will be no Rb gene product at all and the cell will grow abnormally because the growth suppressor is no longer present. The product of the Rb gene has been identified and shown to be a nucleus-located protein of 105 kDaltons. A heterozygote at the Rb allele still has normal Rb and tumors can still be suppressed but homozygote has no functional Rb and tumors cannot be suppressed
|
|||||||||||||||||||||||||
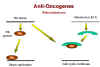 Rb and adenovirus
E1A
Rb and adenovirus
E1A Figure 25 |
Above, we have noted that the adenovirus E1A (early function) protein is somehow involved in tumorigenesis. It has been found that E1A protein in the transformed adenovirus-infected cell is complexed with a 105kD protein! This turns out to be the Rb gene product (Figure 25). Thus, it seems that adenovirus may cause a cell to grow abnormally by complexing (and thereby inactivating) a cellular protein whose normal function is growth inhibition. | |||||||||||||||||||||||||
|
p53 and human cancer Over the past two decades, since its discovery in 1979, a gene known as the p53 gene (after the size of its encoded protein) has been linked to many cancers including many that are inherited. In these inherited cancers, it turns out that the p53 gene is mutated. Alterations in this protein seem to be the basis (direct or indirect) of most human cancers. In total, 60% of human cancers involve p53. 80% of colon cancers involve the p53 gene
|
||||||||||||||||||||||||||
|
Initially, it was thought that the p53 gene product caused cancers but further investigation showed the opposite; p53 is, like the retinoblastoma gene product, a tumor suppressor. p53 protein has been referred to as The Guardian of the Genome since it regulates multiple components of the DNA damage control system. How does p53 work in a functional cell? Normally, there are only a few of the suppressor p53 molecules in a healthy cell and these are constantly turning over; but when the DNA becomes damaged (perhaps by radiation or chemical mutagens) and DNA replication results, p53 turnover ceases. The rise in p53 stops DNA replication. p53 is a transcription factor. When it builds up, p53 binds to a specific site(s) on the chromosomes and switches on other genes and these, in turn, shut down mitosis. p53 can also act in another way: When it builds up it can set the cell on course to apoptosis. Whether or not p53 causes reversible growth arrest or apoptosis depends on the state of cellular activation; for example, extensive, unrepaired DNA damage can lead to sustained p53 production committing the cell to apoptosis. In inherited cancers, there is a mutation in the p53 gene; often it is a single point mutation and the protein can no longer bind to its correct site on the DNA and so cannot suppress DNA replication. Like the Rb gene, product, you would expect the effects of p53 to be recessive since the second normal p53 allele should make functional protein and should shut off DNA replication as usual; however, if you are heterozygous for the mutation, you are, of course, only one mutation away from carcinogenesis. So why do cells that are heterozygous for the p53 mutation also have problems? Unfortunately, p53 protein forms tetramers in a ribbon-like array and so if half of the p53 proteins are mutant, there is a good chance that each tetramer will have one mutant p53 molecule and this inactivates the tetramer, a dominant-negative effect.
|
||||||||||||||||||||||||||
 p53, hepatitis C
and papilloma virus
p53, hepatitis C
and papilloma virus Figure 26 |
Although we have learned a lot from families that inherit p53 mutations, it is clear that most p53 mutations come from non-inherited environmental factors: carcinogens (benzopyrene in smoke, aflatoxin in molds on peanuts and corn, UV light) that result in point mutations. There are also gain of function p53 mutations that lead to very aggressive tumors. These turn on DNA replication genes. What has this got to do with DNA tumor viruses? Just as with retinoblastoma gene product, the presence of a virus can mimic mutation and take the tumor suppressor out of action by complexing it in an inactive form that cannot bind to the specific site on DNA. This is what appears to happen in hepatitis C which causes hepatocellular carcinoma. In the case of a human papilloma virus-infected cell, p53 is bound by the E6 protein and directed to a protease that recognizes a cleavage site in p53, thereby destroying it (Figure 26). In addition, E7 protein binds and inactivates Rb protein. Much research is now going on to see whether one can introduce healthy p53 genes into cells to shut down tumor growth. Thus, our knowledge of how retroviruses cause cancer has led to an understanding of the formerly cryptic manner in which DNA tumor viruses do the same thing.
|
|||||||||||||||||||||||||
|
||||||||||||||||||||||||||