|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
RNA tumor viruses have been moved to a separate page
Follow Next below |
VIROLOGY - CHAPTER SIX
PART ONE
ONCOGENIC VIRUSES
DNA Tumor Viruses
Dr Richard Hunt
Professor
Department of Pathology, Microbiology and Immunology
University of South Carolina School of Medicine
|
|
|
|
EN
FRANCAIS |
|
En
Español |
|
NË SHQIPTARE |
|
TURKISH |
|
Let us know what you think
FEEDBACK |
|
SEARCH |
|

 |
|
|
|
|
|
TEACHING
OBJECTIVES
To learn which viruses can cause cancer in humans
To
learn how cells become transformed by the virus
To
learn the differences between DNA and RNA tumor viruses
To
understand how RNA viral oncogenes result in cell transformation
|

Cancers are the result of a disruption of the normal restraints on cellular proliferation. It is
apparent that the number of ways in which such disruption can occur is strictly limited and
there may be as few as forty cellular genes in which mutation or some other disruption
of their expression leads to unrestrained cell
growth.
There are two classes of these genes in which
altered expression can lead to loss of
growth control:
- Those genes that are stimulatory for growth and which cause cancer
when hyperactive. Mutations in these genes will be dominant. These genes
are called oncogenes.
- Those genes
that inhibit cell growth and which cause cancer when they are turned off. Mutations
in these genes will be recessive. These are the anti-oncogenes or
tumor-suppressor genes.
Viruses are involved in cancers because they can
either carry a copy of one of these genes or can alter expression of the cell's
copy of one of these genes. These are the oncogenic virus (otherwise known as
oncoviruses or tumor viruses).
|
|
To
understand the discovery of cellular proto-oncogenes
To
learn how cellular oncogenes may cause cancer in the absence of a virus
To
learn understand how these discoveries led to the discovery of anti-oncogenes
To
understand how the discovery of anti-oncogenes showed how DNA viruses
may cause cancer |
CLASSES OF TUMOR VIRUSES
There are two classes of tumor viruses:
- DNA tumor viruses
- RNA
tumor viruses, the latter also being referred to as RETROVIRUSES.
We shall see
that these two classes have very different ways of reproducing themselves but they
often
have one aspect of their life cycle in common: the ability to integrate
their own genome into that of the host cell. Such integration is not, however, a
pre-requisite for tumor formation.
TRANSFORMATION AND ONCOGENES
If a virus takes up residence in a cell and alters the properties of that cell, the
cell is said to be transformed. Transformation by a virus is the change in the
biological properties of a cell that results from the regulation of the cell by
viral genes and that confer on the infected cells certain properties of
neoplasia.
Transformation often includes loss of
growth control,
anchorage-independent growth, ability to invade
extracellular
matrix, dedifferentiation and
immortalization. In carcinomas, many epithelial cells undergo an
epithelial-mesenchymal transformation. Transformed cells often exhibit chromosomal
aberrations and the changes seen in transformation often, but not always, result
from the integration of the viral genome into the host cell's chromosomes.
The region of the viral genome (DNA in DNA tumor-viruses or RNA in RNA-tumor viruses)
that can cause a tumor is called an oncogene. This foreign gene can be carried into
a cell by the virus and cause the host cell to take on new properties.
The discovery of viral oncogenes in retroviruses led to the finding
that they are not unique to viruses and homologous genes (called proto-oncogenes)
are found in all cells. Indeed, it is likely that the virus picked up a cellular
gene during its evolution and this gene has subsequently become altered. Normally, the cellular
proto-oncogenes are not expressed in a quiescent cell since they are involved in
growth (which is not occurring in most cells of the body) and development; or they are
expressed under strict control by the cell. However, they may become aberrantly expressed when the cell is
infected by tumor viruses that do not themselves carry a viral oncogene.
We shall see later how this happens but it is clear that a virus may cause
cancer in two ways: It may carry an oncogene into a cell or it may activate a cellular
proto-oncogene.
The discovery of cellular oncogenes opened the way to the elucidation
of mechanisms by which non-virally induced cancers may be caused. We shall
investigate what the protein products of the viral and cellular oncogenes do in the
infected cell and in cells in which cellular proto-oncogenes are expressed. We
shall see that their functions strongly suggest mechanisms by which cells may be
transformed to a
neoplastic phenotype. The discovery of cellular oncogenes led to
the discovery of another class of cellular genes, the tumor repressor (suppressor)
genes or anti-oncogenes.
Initially, the involvement of viral and cellular oncogenes in tumors caused by
retroviruses was much more apparent than the involvement of the DNA tumor virus oncogenes
but the discovery of tumor repressor genes (as a result of our knowledge of how
retroviruses cause cancer) led to the elucidation of the mode of action of DNA virus
oncogenes.
It should be noted that while retroviruses have been instrumental in elucidation of
the mechanisms of oncogenesis, most human cancers are probably not the result of a
retroviral
infection although retroviruses are important in cancers in some animals. It is
becoming much more apparent that many human tumors may result from infection by
DNA tumor viruses.
|
 Figure 1
Figure 1
The information flow in DNA tumor viruses is similar to that in eucaryotic
cells
|
| Figure 2
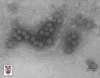 Papilloma virus Copyright 1994 Veterinary Sciences
Division, Queens University Belfast
Papilloma virus Copyright 1994 Veterinary Sciences
Division, Queens University Belfast |
DNA TUMOR VIRUSES
DNA tumor virus have a DNA genome that is transcribed into RNA which
is translated into protein (figure 1). They have two life-styles:
- In permissive cells, all parts of the viral genome are expressed. This leads
to viral replication,
cell lysis and cell death
- In cells that are non-permissive for replication, viral DNA is
usually, but not always, integrated into the cell
chromosomes at random sites. Only part of the viral genome is
expressed. This is the early, control functions (e.g. T antigens) of the virus. Viral structural
proteins are not made and no progeny virus is released.
|
|
 Papilloma virus Copyright Dr
Linda M Stannard, 1995
(used with permission)
Papilloma virus Copyright Dr
Linda M Stannard, 1995
(used with permission)
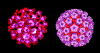 Papilloma virus Computer colorized EM image. All 72 capsomeres are pentamers of the major structural
protein. Copyright
Dr
Linda M Stannard, 1995
(used with permission)
Papilloma virus Computer colorized EM image. All 72 capsomeres are pentamers of the major structural
protein. Copyright
Dr
Linda M Stannard, 1995
(used with permission)
 Figure 3A
Figure 3A
Venereal warts in the anal region of the perineum.
Condylomata acuminata, or genital warts, is a sexually
transmitted disease caused by the Human Papilloma Virus, (HPV)
CDC |
DNA TUMOR VIRUSES INVOLVED IN HUMAN CANCERS
The first DNA tumor viruses to be discovered were rabbit
fibroma virus and Shope
papilloma virus, both discovered by Richard Shope in the 1930s.
Papillomas are benign growths, such as warts, of epithelial cells. They
were discovered by making a filtered extract of a tumor from a wild
rabbit and injecting the filtrate into another rabbit in which a benign
papilloma grew. However, when the filtrate was injected into a domestic
rabbit, the result was a
carcinoma, that is a malignant growth. A seminal observation was
that it was no longer possible to isolate infectious virus from the
malignant growth. This was because the virus had become integrated into
the chromosomes of the malignant cells.
SMALL DNA TUMOR VIRUSES
FAMILY: PAPILLOMAVIRIDAE
PAPILLOMA VIRUSES
The Papillomaviridae were formerly classified with the Polyomaviridae
within the family Papovaviridae (so named for Pa: papilloma; Po:
polyoma; Va: vacuolating). This term is no longer used, the papillomas
and polyomas now being considered separate families.
The papillomaviridae are small non-enveloped icosahedral DNA viruses
(figure 2).
The major capsid protein, L1, is present as 72 pentamers (capsomers).
This protein is all that is required to form the icosahedral capsid
which occurs by self assembly. Each pentamer
is associated with one molecule of another minor capsid protein, either
L2 or L3. Papilloma viruses have a genome size about 8 kilobases
and the DNA is complexed with histone proteins encoded by the
host cell.
These viruses cause warts (figure 3A) and also human
and animal
cancers. Warts are usually benign but can convert to malignant carcinomas. This occurs in
patients with epidermodysplasia verruciformis (figure 3B).
Epidermodysplasia verruciformis
is
also known as Lewandowsky-Lutz dysplasia or Lutz-Lewandowsky
epidermodysplasia verruciformis and is very rare. It is an autosomal
recessive mutation that leads to abnormal, uncontrolled papilloma virus
replication. This results in the growth of scaly macules and papules on
many parts of the body but especially on the hands and feet. Epidermodysplasia verruciformis,
which is associated with a high risk of skin carcinoma,
is typically associated with HPV types 5 and 8 (but other types may also
be involved). These infect most people (up to 80% of the population) and
are usually asymptomatic.
Papilloma viruses are also
found
associated with human penile, uterine, cervical and anal carcinomas and are very likely to be
their cause; moreover, genital warts can convert to carcinomas.
Squamous cell carcinomas of larynx, esophagus and lung appear very like cervical
carcinoma histologically and these may also involve papilloma viruses. Recently,
a strong causal link between certain oral-pharyngeal cancers and HPV16 has been
demonstrated.
There are more than 100 types of human papilloma viruses but, clearly, not all are associated with
cancers; however, papillomas may cause 16% of female cancers worldwide and 10% of all
cancers.
|
| Figure 3B
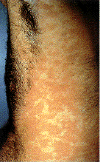 Epidermodysplasia verruciformis. This widespread, markedly
pruritic, erythematous eruption was eventually found to be caused by human papillomavirus infection.
International Association of Physicians in AIDS Care
Epidermodysplasia verruciformis. This widespread, markedly
pruritic, erythematous eruption was eventually found to be caused by human papillomavirus infection.
International Association of Physicians in AIDS Care

Epidermodysplasia verruciformis:
Hyperkeratotic warty lesions on
dorsal aspect of hands

Epidermodysplasia verruciformis:
Histopathological view: Koiliocytes and moderate dysplasia in the
epidermis (H&E x100)
From: Reza Mahmoud Robati MD, Afsaneh Marefat MD, Marjan Saeedi MD,
Mohammad Rahmati-Roodsari MD, Zahra Asadi-Kani MD
Dermatology Online Journal 15 (4): 8, 2009 (used under Creative Commons
license)
 Verrucous carcinoma. The epithelium shows surface maturation,
parakeratosis, and hyperkeratosis. There is little or no cellular atypia. The stroma shows a mild chronic inflammatory infiltrate.
The Johns Hopkins Autopsy Resource
(JHAR) Image Archive.
Verrucous carcinoma. The epithelium shows surface maturation,
parakeratosis, and hyperkeratosis. There is little or no cellular atypia. The stroma shows a mild chronic inflammatory infiltrate.
The Johns Hopkins Autopsy Resource
(JHAR) Image Archive.
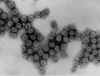 Figure 4A Figure 4A
Transmission electron micrograph of polyomavirus SV40
Dr. Erskine Palmer CDC
 Figure 4B
Figure 4B
Human polyomaviruses and associated diseases.
The organs to which each human polyomavirus has tropism and causes
disease.
doi:10.1371/journal.ppat.1003206.g001
From: The Rapidly Expanding Family of Human Polyomaviruses: Recent
Developments in Understanding Their Life Cycle and Role in Human
Pathology. Martyn K. White, Jennifer Gordon and Kamel Khalili.
PLOS Pathogens. Used under Creative Commons License
|
Vulvar, penile and cervical cancers are associated with type 16 and type 18
papilloma viruses (and others) but the most common genital human papilloma viruses
(HPV)
are types 6 and 11. As might be expected if they are indeed the causes of certain
cancers, types 16 and 18 cause transformation of human keratinocytes. In a German study,
it was shown that 1 in 30 HPV type16-infected women will develop malignant disease while 1 in 500
infected people develop penile or vulvar cancer. Since not all infected persons develop a
cancer, there are probably co-factors in stimulating the disease. Such co-factors have
been identified in alimentary tract carcinomas in cattle where a diet containing bracken
fern is associated with the disease. People with HIV infection or AIDS are at increased risk of HPV-associated
cancers as are patients with other forms of immunosuppression.
The fact that a virus is usually found in association with a disease
(often, in the case of tumors, the presence of a copy of the viral genome in
the neoplastic cells) does not prove that the virus caused the cancer. The
association could be casual rather that causal. Nevertheless,
in many instances the epidemiological data are very strong
and, in the case of human cervical cancer, the efficacy of the anti-HPV vaccine
makes the contention that HPV does cause cervical cancer very compelling.
FAMILY: POLYOMAVIRIDAE
POLYOMA VIRUSES
The polyomaviridae (figure 4A) are small non-enveloped icosahedral DNA
viruses (figure 2). The major capsid protein, VP1, is present as 72
pentamers. Each pentamer is associated with one molecule of another minor
capsid protein, either VP2 or VP3. They have a genome of about 5 kilobases.
Each particle is about 40--50 nanometers across.
Until recently, there was only one genus of polyoma viruses. However,
more have been discovered and in 2010, the single genus was split into
three:
- Orthopolyomavirus This contains the classic mammalian polyomaviruses
(e.g., JCPyV, BKPyV, SV40, mouse polyomavirus, etc.);
- Wukipolyomavirus This contains the recently discovered human
polyomaviruses including Karolinska Institute polyomavirus (KIPyV) and
the Washington University polyomavirus (WUPyV);
- Avipolyomavirus. This contains the avian polyomaviruses
Many polyoma viruses have been associated with human disease (figure 4b).
Mouse (Murine) Polyoma virus
Polyoma virus was so named because it causes a wide range of tumors in a
number of animal species at many different sites. It was originally isolated from AK mice and is fully
permissive for replication in mouse cells. It causes leukemias in mice and
hamsters.
Simian virus 40
SV40 virus was initially discovered in
the rhesus monkey kidney cells that were used to make inactivated Salk polio
vaccine virus. It was found that when the inactivated polio virus made in
these cells was added to African Green Money Kidney cells, the vaccine gave
a cytopathic effect indicative of the presence of a live virus that
had not been killed by the formalin used to inactivate the vaccine virus.
SV40 replicates in rhesus monkey kidney cells but has no cytopathic effect
on them. Many early recipients of the Salk polio vaccine received
contaminating SV40 since anti-SV40 antibodies (against a protein called the
large tumor antigen (T-antigen)) could be detected in their
blood. No elevated incidence of cancer has been found in these people.
Although
SV40 is a monkey virus that has no apparent effect on the host animal, it
causes sarcomas when injected into juvenile hamsters. The hamster tumor
cells produce no infective virus.
Human polyoma viruses
The first two human polyoma isolates, known as BK and JC
were discovered in 1971.
Neither came from a tumor. BK came from the urine of a kidney transplant patient
and JC came from the brain of a Hodgkin's lymphoma patient who progressed to
progressive multifocal leukoencephalopathy (PML); however, they
cause tumors when injected into animals. 70 to 80% of the human population is seropositive for JC. This virus
is known to be the cause of PML (see
slow viral diseases), a disease associated
with immunosuppression. In 1979, the rate of occurrence of this disease was 1.5
per 10 million population. It has become much more common because of AIDS and is
seen in 5% of AIDS patients. BK virus is an important cause of nephropathy and
graft failure in immuno-suppressed renal transplant recipients and almost
everyone in western countries has anti-BK virus antibodies by the age of 10.
Recently, BK viral DNA has been associated with human prostate cancer.
Three other human polyoma viruses have recently been described: KI, WU and Merkel cell
polyoma virus. The latter virus causes a rare skin cancer (Merkel cell
carcinoma, see box below).
|
|
WEB RESOURCES
Cutaneous
manifestations of human papilloma virus
Epidermodysplasia
verruciformis
E-medicine
Human papilloma vaccine
CDC |
|
Figure 5
 Adenovirus
Adenovirus
Copyright
Dr Stephen
Fuller, 1998

Adenovirus
CDC
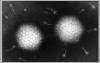 Adenovirus
Copyright
Dr
Linda
M Stannard, University of Cape Town, South Africa, 1995
(used with permission). Adenovirus
Copyright
Dr
Linda
M Stannard, University of Cape Town, South Africa, 1995
(used with permission).
|
Polyoma viruses are usually lytic (cause
lysis) and when transformation occurs, it is
because the transforming virus is defective. After integration into host DNA, only
early functions are transcribed into mRNA and expressed as a protein product. These
are the tumor antigens. Because the expression of the genes for tumor antigens is
essential for transformation of the cells, they may be classified as oncogenes.
DEFINITION OF AN ONCOGENE: AN ONCOGENE IS A GENE THAT CODES FOR A PROTEIN THAT
POTENTIALLY CAN TRANSFORM A NORMAL CELL INTO A MALIGNANT CELL. IT MAY BE TRANSMITTED BY A
VIRUS IN WHICH CASE WE REFER TO IT AS A VIRAL ONCOGENE.
FAMILY: ADENOVIRIDAE
ADENOVIRUSES
These viruses (figure 5) are somewhat larger than polyoma
and papilloma viruses with a genome size of about 35 kilobases. They were
originally isolated from human tonsils and adenoids, are highly oncogenic in animals and only a portion of the virus is
integrated into the host genome. This portion codes several T antigens
that carry out early functions. Tumor-bearing animals make antibodies
against the T antigens.
No humans cancers have been unequivocally
associated with adenoviruses.
|
| |
Tumor antigens are oncogenes
Tumors caused by papilloma virus, adenovirus or polyoma virus contain viral
DNA but do not produce infectious virus. The presence of the virus, however,
elicits the formation of antibodies against the tumor antigens. In the case of
adenoviruses, only part of the viral genome is found in the host cell
chromosomes whereas SV40 may integrate part or all of its genome. Whether or not
the whole SV40 genome is integrated, only a part of the genome is transcribed
into mRNA and protein and this is the region that encodes the early functions of
the virus replication cycle.
Many DNA
viruses have early and late functions. Early functions are the result of the
expression of proteins that prime the cell for virus production and are involved
in viral DNA replication. These proteins are expressed before genome replication
and do not usually end up in the mature virus particle. Late functions are the
results of the expression of viral structural proteins that combine to form the
mature virus. They are expressed during and after the process of DNA
replication. Since early functions are involved in the replication of the viral
genome, it is not surprising that they can also alter the replication of host
cell DNA.
SV40 expresses two such proteins, the T antigens (large T and small T
antigen). The large T antigen
acts as a cis-regulatory element at the level of viral DNA replication by
binding to the origin of replication and stimulating transcription. It can also
bind to and modulate the activity of host cell DNA polymerase alpha.
As we shall see later, DNA replication in the cell is controlled by
suppressor proteins (the best studied of which are the retinoblastoma (Rb) and
p53 suppressor proteins). SV40 large T antigen can bind directly to these proteins and
inactivate them, thereby inducing the cell to go from Go to S phase. Because polyoma
viruses have a small genome, they rely on many cell functions for DNA
replication and it is important that the virus causes the cell to enter S phase
because it creates a suitable environment for viral DNA replication.
Thus, SV40 Large T antigen:
- is necessary for transformation of a cell to
the cancerous state
- stimulates the host cell to replicate its DNA
- is found mostly in the nucleus (to
which it is directed by its nuclear localization signal) but a
small amount goes to the cell surface (where it is a tumor-specific transplantation antigen)
- binds to cellular DNA
- binds to p53 protein (see below)
A second T antigen (small T antigen) interacts with a family of cellular
phosphatases (called pp2A) which results in the failure of certain cellular
proteins to be phosphorylated, thereby relieving cell cycle arrest.
In mouse polyoma virus, there is a middle T antigen which can
also act as an oncogene.
Similarly, in adenovirus-induced tumors, only a part of the viral genome
becomes integrated and again it is the early region genes. This region codes for
the E1A and E1B proteins. In papilloma virus-induced tumor, again, two early
genes, E6 and E7, are expressed.
Thus, papilloma, polyoma and adenoviruses seem to cause cell
transformation in a similar manner: the integration of early function genes into the
chromosome and the expression of these DNA synthesis-controlling genes without the
production of viral structural proteins.
As we shall see later, all three virus types induce cell proliferation by
interacting with tumor suppressor genes.
Two important points that should be emphasized about T antigens of DNA tumor viruses as oncogenes:
- They are true viral genes. There are no cellular homologues in the
uninfected cell
- They are necessary in lytic infections because they participate in the control
of viral and cellular DNA transcription
These properties should be contrasted with retroviral oncogenes to be discussed later
|
|
Figure 6
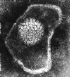 Herpes virus. Negative stain Copyright Dr
Linda M Stannard,
University of Cape Town, South Africa, 1995 (used
with permsssion).
Herpes virus. Negative stain Copyright Dr
Linda M Stannard,
University of Cape Town, South Africa, 1995 (used
with permsssion).
 Liquid-Crystalline, Phage-like Packing of Encapsidated DNA in Herpes Simplex Virus
(F.P.Booy, W.W.Newcomb, B.L.Trus, J.C.Brown, T.S.Baker,
and A.C.Steven, in CELL, Vol 64 pp 1007-1015, March 8, 1991)
Liquid-Crystalline, Phage-like Packing of Encapsidated DNA in Herpes Simplex Virus
(F.P.Booy, W.W.Newcomb, B.L.Trus, J.C.Brown, T.S.Baker,
and A.C.Steven, in CELL, Vol 64 pp 1007-1015, March 8, 1991)
 Herpes Simplex Virus (TEM x169,920)
©
Dennis Kunkel Microscopy, Inc.
Used with permission
Herpes Simplex Virus (TEM x169,920)
©
Dennis Kunkel Microscopy, Inc.
Used with permission
|
COMPLEX TUMOR VIRUSES
FAMILY: HERPESVIRIDAE
HERPESVIRUSES
Herpesviruses (figure 6) are much larger than the DNA viruses described
above and have a genome size of 100 to 200 kilobases. Because of
their large size, a lot remains to be discovered concerning the way
in which these viruses transform cells.
There is considerable circumstantial evidence that implicates these
large enveloped viruses in
human cancers and they are highly tumorigenic in animals. The herpes virus genome
integrates into the host cell at specific sites and may cause chromosomal
breakage or other damage (see below). Herpesviruses are often co-carcinogens. They
may have a hit and run mechanism of oncogenesis, perhaps by expressing proteins
early in infection that lead to chromosomal
breakage or other damage.
Herpesviruses have over 100 genes. When these
viruses infect cells which are non-permissive for virus production but
which are transformed, only a subset (about 9) of viral genes are
expressed. These genes code of nuclear antigens or membrane proteins.
Not all nine transformation-associated genes are expressed in all
herpes-transformed cells.
Epstein-Barr virus (Human herpes virus 4)
EBV (figure 7A) is the herpes virus that is most strongly associated
with cancer. It infects primarily lymphocytes and epithelial cells. In
lymphocytes, the infection is usually non-productive, while virus is shed
(productive infection) from infected epithelial cells.
EBV is causally associated with:
- Burkitt's lymphoma (figure 7B) in the tropics
(figure 7C), where it is
more common in malaria-endemic regions
- Nasopharyngeal cancer, particularly in China
and SE Asia, where certain diets may act as co-carcinogens
- B cell lymphomas in immune suppressed individuals (such as in organ transplantation or
HIV)
- Hodgkin's lymphoma in which it has been detected in
a high percentage of cases (about 40% of affected
patients)
- X-linked lymphoproliferative Disease (Duncan's syndrome)
EBV can cause lymphoma in Marmosets and transform human B lymphocytes in vitro.
EBV also causes infectious mononucleosis, otherwise
known as glandular fever (figure 7D). This is a self-resolving infection of
B-lymphocytes which proliferate benignly. Often infection goes unnoticed (it
is sub-clinical) and about half of the population in western countries has
been infected by the time they reach 20 years of age. Why this virus causes a benign
disease in some populations but malignant disease in others is unknown.
|
 Figure 7A
Figure 7A
Epstein- Barr Virus
|
 Figure 7B
Figure 7B
Burkitt's Lymphoma caused by Epstein-Barr Virus
The Johns Hopkins Autopsy Resource
(JHAR) Image Archive.
 Figure 7C
Figure 7C
Distribution of Burkitt's lymphoma
 A
A
 B
B
Figure 7D
Peripheral blood smears from a healthy individual (A)
and a patient with infectious mononucleosis caused by Epstein-Barr virus
(EBV)
(B). Both smears are stained with Giemsa stain
©
Gloria J. Delisle and Lewis Tomalty Queens University
Kingston, Ontario, Canada and
The
MicrobeLibrary
|
 Figure 7E
Figure 7E
Early oral hairy leukoplakia (OHL) on the lateral border
of the tongue.
HIV reduces immunologic activity, the intraoral environment is a prime
target for chronic secondary infections and inflammatory processes,
including oral hairy leukoplakia, which is due to the Epstein-Barr virus
under immunosuppressed conditions
CDC
 Figure 7F
Figure 7F
Transmission electron micrograph of cytomegalovirus
virions (x49,200)
CDC |
Human Herpes Virus 8 (HHV-8, Kaposi's Sarcoma Herpes Virus)
HHV-8 infects lymphocytes and epithelial/endothelial cells and is the
causative agent of Kaposi's sarcoma. It has also been associated with
hematologic malignancies, including primary effusion lymphoma, multicentric Castleman's (also
Castelman's) disease (MCD), MCD-related immunoblastic/plasmablastic lymphoma and
various atypical lymphoproliferative disorders.
EBV and HHV-8 have been found to be associated with oral lesions and
neoplasms in HIV-infected patients. Among these diseases is oral hairy
leukoplakia (OHL, figure 7E) which is benign and causes white thickenings on the tongue
epithelium in which these viruses proliferate.
Human cytomegalovirus (Human Herpes Virus 5)
This herpes virus (figure 7F) is frequently associated with
Kaposi's sarcoma but this disease is now thought probably to be caused by human herpes virus 8.
For more on herpes viruses and the diseases that
they cause, go to Virology Chapter 11
Herpes
Viruses
|
|
Figure 8
 This woman has hepatitis B and is suffering from liver cancer. She was a Cambodian refugee
and died 4 months after she arrived in a refugee camp (average life expectancy after diagnosis of liver cancer is 6 months)
Immunization Action Coalition Courtesy of Patricia Walker, MD, Ramsey Clinic Associates, St. Paul, MN
This woman has hepatitis B and is suffering from liver cancer. She was a Cambodian refugee
and died 4 months after she arrived in a refugee camp (average life expectancy after diagnosis of liver cancer is 6 months)
Immunization Action Coalition Courtesy of Patricia Walker, MD, Ramsey Clinic Associates, St. Paul, MN
|
FAMILY: HEPADNAVIRIDAE
HEPATITIS B VIRUS
Hepatitis B virus (figure 9) is very different from the other DNA tumor viruses.
Indeed, even though it is a DNA virus, it is much more similar to the oncornaviruses (RNA tumor viruses) in its mode of replication. The DNA is
transcribed into RNA not only for the manufacture of viral proteins but
for genome replication. Genomic RNA is transcribed back into genomic
DNA. This is called reverse transcription. The latter is not typical of
most DNA
tumor viruses but reverse transcription is a very important factor in
the life cycles of RNA-tumor viruses. See below.
For more information on the molecular
biology of hepatitis B virus and the diseases it causes, go to
chapter 18
and chapter 19, part 2.
Hepatitis B is a vast public health
problem and hepatocellular carcinoma (HCC) (figure 8), which is one of world's most common cancers, may well be caused by HBV. There is a very strong correlation between HBsAg (hepatitis B virus surface
antigen) chronic carriers and the incidence of HCC. In Taiwan, it
has been shown that HBsAg carriers have a risk of HCC
that is 217 times that of a non-carrier. 51% of deaths of HBsAg carriers are caused by
liver cirrhosis or
HCC compared to 2% of the general population.
|
Figure 9
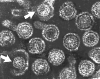 Hepatitis B virions: two exposed cores (indicated by
arrows)
Hepatitis B virions: two exposed cores (indicated by
arrows) |
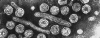
 Hepatitis B virions
Hepatitis B virions
 A diagrammatic representation of the hepatitis B virion and the surface antigen
components
A diagrammatic representation of the hepatitis B virion and the surface antigen
components
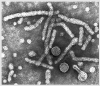
 Hepatitis B Virus
Hepatitis B Virus
All four images: Copyright
Dr
Linda M Stannard,
University of Cape Town, South Africa,
1995 (used with permission).
|
|
|
|
|


|
 Return to the Virology Section of Microbiology and Immunology On-line
Return to the Virology Section of Microbiology and Immunology On-line
 Return to the
front page of Microbiology and Immunology On-line Return to the
front page of Microbiology and Immunology On-line
This page last changed on
Sunday, June 05, 2016
Page maintained by
Richard Hunt
|


 Figure 1
Figure 1 Figure 7A
Figure 7A Figure 7E
Figure 7E Hepatitis B virions: two exposed cores (indicated by
arrows)
Hepatitis B virions: two exposed cores (indicated by
arrows)






















