Figure 1a
Figure 1a
Gram-stained Gram-positive Clostridium tetani bacteria, which had
been cultivated on a blood agar plate. C. tetani is a slender,
anaerobic rod that may develop a terminal spore, giving it a drumstick
appearance. CDC/Dr Holdeman
 Figure 1b
Figure 1b
C. tetani. Note terminal spores. CDC
 Figure 2a
Figure 2a
Tetanus cases by age 1980-2000 CDC
 Figure 2b
Figure 2b
Age distribution of reported tetanus cases 1991-1995 and 1996-2000 CDC
 Figure 3
Figure 3
Annual rate of tetanus cases and tetanus deaths in the United States during
1947–2008. From 1947–2008, the number of tetanus cases reported each year, which
already had decreased greatly since 1900, continued to decline. CDC
|
Tetanus
Clostridium
tetani
Clostridium tetani, a gram-positive rod
that forms a terminal spore (figure 1a and b), is commonly found in the
soil, dust and animal feces. Contamination of wounds, which provide anaerobic
conditions, can lead to spore germination and tetanus, a
relatively rare (in western countries) but frequently fatal disease. Death
occurs in about 11% of cases with most of these in the more elderly patients
(over 60 years of age). Tetanus is
also know as lockjaw because of the patient's inability to open the mouth as a
result of muscle paralysis. The rarity of the disease results from an excellent
vaccine and most cases that are now seen in the United States are in adults who never
received the vaccine. Thus, currently some 60% of cases are in adults over 50
(figure 2). In the
period 1947 to 2008, tetanus cases in the US dropped by over 95% and deaths by
over 99% (figure 3). From 2000 through 2009 an average of 29 cases were
reported per year in the United States. Vaccination has reduced neonatal tetanus in developed countries so
that in the United States there have been just two cases since 1989. Both
patients were born to unvaccinated mothers.
In third world countries, many (about half) of tetanus cases are
in neonates where the unhealed umbilical stump becomes infected, often as a
result of cutting the umbilical cord with a contaminated knife. Many neonatal deaths
result (about 270,000 in 1998). This occurs when the mother has no protective
immunity to pass on to the infant
Infection usually occurs when spores
(in dirt, feces or saliva) enter wounds and scratches where they germinate and produce tetanus toxin.
Puncture wounds, such as by a needle or nail, other wounds and scratches and
burns can all lead to C. tetani tinfections. More rarely, surgical
procedures and dental extractions can lead to tetanus. Tetanus can also be
contracted from the use of intravenous drugs.
The organism is non-invasive and
thus remains in the local wound. The exotoxin (tetanospasmin) binds
to ganglioside receptors on inhibitory neurones in
central nervous system in which
glycine is
commonly the neurotransmitter. This stops nerve impulse transmission to muscle
leading to spastic paralysis. The toxin can act at peripheral motor nerve end
plates, the brain, spinal cord and also in the sympathetic nervous system.
Because inhibitory neurons are involved, the result is unopposed muscle
contraction.
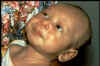
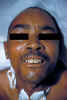
In generalized tetanus, the most
common form, the patient typically experiences lockjaw (trismus).
This is a stiffness of the jaw muscles that results in inability to open the mouth
(figure 4) or
swallow leading to the appearance of a sardonic smile (risus sardonicus)
(figure 5). Speech
as a result of spasm of the vocal cords may be affected. Continued severe muscle contractions
(figure 6 and 7),
which can even cause broken bones, and resulting spasms, often lasting for
minutes over a period of weeks, can be fatal. The patient often experiences
headaches and/or a
fever (a rise of 2 to 4 degrees) with sweating, elevated heart rate and blood
pressure. Tetanus patients in hospital often experience nosocomial
infections. Aspiration pneumonia is often a late complication.
On average, about eight days after
infection symptoms of tetanus appear (though the incubation period can be a
short as three days and as long as thee weeks). The incubation period seems to depend
on the distance of the infection site from the central nervous system. In
neonates the average latent period is about a week.
Other forms of tetanus
Cephalic tetanus is a rare infection
involving the middle ear. It can affect cranial nerves.
Local tetanus is also rare and manifests
itself as localized muscle contractions in the area of infection. Few cases of
local tetanus are
fatal.
Vaccination
Vaccination of infants with tetanus
toxoid have almost eliminated this disease in the United States. The toxoid
consists of tetanus toxin that has been inactivated using formalin that
stimulates anti-toxin antibodies. It comes in two forms: precipitated and fluid.
The precipitated form yields more rapid seroconversion and higher anti-toxin
titers. If infants receive the complete vaccination regimen, virtually 100%
protection is achieved. Boosters should be given every ten years.
Diagnosis
Diagnosis is clinical and bacteria are
only derived from wounds in a minority of cases.
Treatment
Tetanus is an emergency situation
and requires hospitalization. The patient is immediately treated with
human tetanus immune globulin (or equine antitoxin). Drugs can control
muscle spasms. The wound requires aggressive washing and treatment with
antibiotics.
|
|
WEB RESOURCES
CDC
tetanus manual
(requires Acrobat)
CDC
information on botulism
CDC
botulism manual
(requires Acrobat)
Seminal
facts about botulism
(from WHO)
Bacterial
toxins: Friend or Foe (from
CDC)
 Figure 13a
Figure 13a
Gentian violet stain of C. botulinum.
© The
MicrobeLibrary
 Figure 13b
Figure 13b
Clostridium botulinum - rod prokaryote. Vegetative (yellow arrow) and spore
(blue arrow) stages : note the flagella on the vegetative cells. Causes botulism.
SEM x15,400, ©
Dennis Kunkel Microscopy, Inc.
Used with permission
 Figure 14
Figure 14
Wound botulism involvement of compound fracture of right arm.
14-year-old boy fractured his right ulna and radius and subsequently
developed wound botulism. CDC
 Figure 15a
Figure 15a
Morphology of Gram-positive Clostridium difficile bacillus. CDC
 Figure 15b
Figure 15b
C. difficile from a stool sample culture. CDC
 Figure
16 Figure
16
Scanning Electron Micrograph of Pseudomonas aeruginosa
CDC
 Figure 17
Figure 17
Three-dimensional computer-generated image of four multidrug-resistant
Pseudomonas aeruginosa bacteria. The artistic recreation was
based upon scanning electron micrographic imagery. Note the presence of
numbers of thin, diaphanous fimbriae emanating from the organisms’ cell
wall, as well as a single, corkscrew-shaped flagellum, which provides
for the bacteria’s unipolar mode of motility.
CDC/Melissa Brower
 Figure 18
Figure 18
Colorized scanning electron micrograph of a number of
Pseudomonas aeruginosa bacteria.
CDC/ Janice Haney Carr
|
Botulism
Clostridium botulinum
Botulism (a rare but fatal form of
food poisoning) is caused by a potent nerve exotoxin (botulinum toxin).
It is a serious paralytic illness caused by Clostridium botulinum
(figure 13) and, more rarely, by strains of Clostridium butyricum
and Clostridium baratii.
The toxin (of which there
are seven types, designated as A through G but only types A, B, E
and F cause illness in humans) binds to receptors on peripheral
nerves, where acetylcholine is the neurotransmitter and inhibits nerve impulses.
Flaccid paralysis and often death (from respiratory and/or cardiac
failure) ensue. The organism
does not grow in the gut, but pre-formed exotoxin from prior germination of
spores may be present in inadequately autoclaved canned food (usually at home).
Besides food poising, C. botulinum can cause:
-
Wound botulism (figure
14) but is
even rarer than botulism food poisoning.
-
In addition,
iatrogenic botulism can occur from accidental overdose of
botulinum toxin.
C. botulinum
does not readily grow in the adult intestine due to competition with the normal
flora and their requirement for an anaerobic, low acidity environment. In
infants, where the flora is not established, colonization with
C.
botulinum can occur. Infant botulism, although uncommon, is now the
predominant form of botulism. In the United States, there are about 150
cases of botulism per year of which three quarters are infant botulism and 15% come from contaminated food
(usually home-reserved food). The remainder are wound botulism
(mostly associated with black-tar heroin injection). The spores can remain viable for many
years.
Symptoms
After eating contaminated
food, the symptoms of botulism occur usually with a day or two but
sometimes there may be a period of up to a week before they appear. Vision
and swallowing are affected and the patient may become nauseated and
constipated. Muscle paralysis ensues, usually starting at the head and,
when the respiratory muscles are affected, death can result. While
the bacterium does not grow in the adult large intestine, it can in
infants who ingest spores that are ubiquitous in the environment
- Eating honey contaminated by spores is one source. Again, an early
symptom is constipation and general malaise. With muscle paralysis,
swallowing becomes difficult and paralysis of the head muscles leads to
the characteristic floppy baby symptoms. Impaired respiratory muscles
lead to breathing difficulties and possibly to death. When severe wounds
are infected, the conditions are right for the growth of clostridia
leading to similar symptoms to food-borne botulism except that the
gastro-intestinal tract is not involved.
Treatment
Treatment for adults includes
an enema to clear the gastro-intestinal tract of the toxin and injection
of anti-toxin (antibodies produced in horses). It is important that the
anti-toxin is given early to neutralize the toxin and protect nerve
endings from damage. The horse-derived anti-toxin is not used in infants
who receive, instead, human botulism immune globulin. Antibiotics are
not used to treat botulism, although they may be used in secondary
infections, because of the possibility of more toxin being released as bacteria
are lyzed. Supportive treatment of infants is based on helping them
breath and on tube feeding. Adults may also require a respirator and
possibly a tracheotomy and intensive medical and nursing care for
several months.
Death from botulism has become much rarer in the
past 50 years. The proportion of patients with botulism who die
has fallen from about 50% to 3 to 5%. Some patients die from
infections or other problems as a result of being paralyzed for
weeks or months. Patients who survive an episode of botulism
poisoning may have fatigue and shortness of breath for years and
long-term therapy may be needed to aid recovery. However,
complete recovery from botulism
usually occurs over a period of months as the damaged nerve endings are
replaced.
Clostridium difficile
C. difficile is frequently a
nosocomial infection. The organism, a gram positive rod (figure
15), can cause a variety of diseases including:
- pseudomembranous colitis, a
form of gastroenteritis
-
toxic megacolon
- perforations of the colon
- sepsis
Patients at elevated risk include those that have
received:
-
antibiotics
-
proton pump inhibitors
-
gastrointestinal surgery/manipulation
-
long length of stay in healthcare settings
-
a serious underlying illness
Those with immunocompromizing conditions and
advanced age are also at high risk
However, C. difficile infection is rarely
fatal.
Symptoms
When the normal flora of the intestine
is altered by antibiotic therapy, this organism - which is present in the
gastro-intestinal tract of many babies - can grow and colonize. C. difficile
produces an
enterotoxin and pseudomembranous colitis can result.
Symptoms, which include abdominal cramps and watery diarrhea, start some days (4
to 8) after initiation of antibiotic therapy. In mild cases, there is no blood
in the diarrhea but, in severe cases, bloody diarrhea, a distended tender
abdomen and fever can occur.
Treatment
Therapy includes
discontinuation of the implicated antibiotic (e.g. ampicillin). Severe cases
require specific antibiotic therapy (e.g. with vancomycin).
PSEUDOMONAS AERUGINOSA
Pseudomonads are
aerobic, gram-negative rods with polar flagella. They are oxidase positive,
in contrast to Enterobacteriaceae. These organisms are found in
most environments including in water and soil and air. Among the genus Pseudomonas,
the majority of human infections are caused by P. aeruginosa (figure 16
and 17), although
other related organisms also cause disease. Normally, individuals with
compromised immune systems such as those infected with HIV, organ
transplant recipients and burns patients are particularly prone to
pseudomonad infections and mortality can be high (e.g. as much as 90% in
heart infections). In burns and wounds,
there is destruction of
blood vessels which limits access of phagocytes that would normally clear the
region of the pathogen. Cystic fibrosis patients are also at risk for
infection since alteration of the
respiratory epithelium commonly allows colonization and
development of pneumonia. This is often seen in children who may suffer
recurrent bouts of pseudomonad pneumonia resulting in fever, a wheezing
productive cough, distended abdomen, breathing difficulties and
cyanosis. This is often accompanied by weight loss.
Pseudomonads are opportunistic
pathogens. Nosocomial infections by P. aeruginosa are
particularly common in intensive care units and can lead to fatal
pneumonia in which the patient has a productive cough, chills, breathing
difficulties and cyanosis. The problem is compounded by the often
encountered resistance of pseudomonads to common antibiotics. Moreover,
the slime layer that is produced over
the surface of these organisms has an anti-phagocytic effect making
their control by the immune system phagocytes difficult; yet, they stick
readily to other cells. They produce tissue-damaging toxins.
Infections by P. aeruginosa
are a common cause of bacteremia, that is bacterial blood
infections. Heart valves, particularly of intravenous drug users, can
also become infected. Symptoms include general malaise with fever with
joint and muscle pain.
Pseudomads can infect the skin
as a result of bathing in infected waters, resulting in a itching rash
in otherwise healthy individuals. This is the so-called "hot tub
folliculitis". Sometimes these skin infections can be severe and
result in headache, sore eyes, stomach and breast pain and earache.
Injury can lead to infections of soft tissues and of bone and joints and
the bacteria can also spread to these sites from a bacteremia. Bone
involvement is sometimes seen in diabetics as well as persons who are undergoing
surgery. Infection of wounds can result in the characteristic fruity
smell and blue-green secretions (pyocyanin).
Among other pseudomonad-caused
infections are those of the urinary tract, often as a result of catheter
use or surgery, the brain which can develop abscesses and meningitis,
and the eyes and ears. Swimmer's itch is an innocuous infection of the
ear canal by these bacteria but older patients can experience
life-threatening infections of the ear which sometimes cause paralysis
of facial muscles. Abrasion of the cornea can lead to infection and
resultant corneal ulcers which, if left untreated, can cause severe
damage and loss of sight. Some eye medications and prolonged use of soft
contact lenses can exacerbate the infection.
Identification of a
pseudomonad infection includes pigment production:
pyocyanin
(blue-green) and fluorescein (green-yellow, fluorescent) and biochemical
reactions (oxidase test). Cultures have fruity smell. Since hospitals
are so commonly infected with pseudomonads, the presence of the organism
is not sufficient to prove it as a source of the infection. Techniques such
as X-rays can be used to assess deep tissue and bone infections.
Resistance of pseudomonads to
various antibiotics is a problem. Two such drugs simultaneously are
often employed for up to 6 weeks, either by mouth or intravenously. Eye
infections are treated with antibiotic drops. In the case of infections
of deep tissues such as in the brain, joints or bone, surgery to remove
damaged tissue may be required. Moreover, amputation may be necessary in
infections of the limbs of burns patients or those with infected wounds.
The toxicity of pseudomonads results from production
of Toxin A which ADP ribosylates elongation factor-2 (EF2 - used
in protein synthesis). In this, pseudomonad toxin is similar to diphtheria toxin
|

 Figure 1a
Figure 1a Figure 13a
Figure 13a