|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
SPANISH |
BACTERIOLOGY - CHAPTER TWENTY
CHLAMYDIA AND
CHLAMYDOPHILA
Dr. Gene Mayer
Professor Emeritus
University of South Carolina School of Medicine
|
|
TURKISH |
|
SLOVAK |
|
ALBANIAN |
|
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|
|
|
|
|
|
|

 |
|
Logo image © Jeffrey
Nelson, Rush University, Chicago, Illinois and
The MicrobeLibrary |
|
TEACHING OBJECTIVES
To describe the developmental cycle of
chlamydia
To describe the pathogenesis, epidemiology and clinical
syndromes associated with chlamydia
KEY WORDS
Elementary bodies
Reticulate bodies
Inclusion
Biovar
Serovar
Trachoma
Inclusion
conjunctivitis
LGV
Reiter's syndrome
Psittacosis
Ornithosis
TWAR agent |
The family Chlamydiaceae consists of
two genera. One species
of Chlamydia
and two of Chlamydophila are important in causing disease in
humans.
-
Chlamydia trachomatis can
cause urogenital infections, trachoma, conjunctivitis, pneumonia and
lymphogranuloma venereum (LGV)
-
Chlamydophila pneumoniae can cause
bronchitis, sinusitis, pneumonia and possibly atherosclerosis
-
Chlamydophila psittaci can cause pneumonia (psittacosis).
Members of the Chlamydiaceae are small obligate
intracellular parasites and were formerly considered to be viruses. However, they
contain DNA, RNA and ribosomes and make their own proteins and nucleic acids and
are now considered to be true bacteria. They possess an inner and outer membrane
similar to gram-negative bacteria and a lipopolysaccharide but do not have a peptidoglycan layer.
Although they synthesize most of their metabolic intermediates, they are unable
to make their own ATP and thus are energy parasites.
Physiology and Structure
Elementary bodies (EB)
EBs are the small (0.3 - 0.4
μm) infectious form of the chlamydia. They possess a rigid outer
membrane that is extensively cross-linked by disulfide bonds. Because of
their rigid outer membrane the elementary bodies are resistant to harsh
environmental conditions encountered when the chlamydia are outside of their
eukaryotic host cells. The elementary bodies bind to receptors on host cells
and initiate infection. Most chlamydia infect columnar epithelial cells but
some can also infect macrophages.
Reticulate bodies (RB)
RBs are the non-infectious
intracellular from of the chlamydia. They are the metabolically active
replicating form of the chlamydia. They possess a fragile membrane lacking
the extensive disulfide bonds characteristic of the EB.
|
|
 Figure 1 The developmental cycle of chlamydia
Figure 1 The developmental cycle of chlamydia
|
Developmental cycle (Figure 1) - The EBs bind to receptors on
susceptible cells and are internalized by endocytosis and/or by phagocytosis.
Within the host cell
endosome the EBs reorganize and become
RBs. The chlamydia
inhibit the fusion of the endosome with the lysosomes and thus resist
intracellular killing. The entire intracellular life cycle of the chlamydia
occurs within the endosome. RBs replicate by binary fission and reorganize
into EBs. The resulting inclusions may contain 100 - 500 progeny (Figure 2). Eventually,
the cells and inclusions lyse (C. psittaci) or the inclusion is
extruded by reverse endocytosis (C. trachomatis and C. pneumoniae)
(Figure 1).
|
|
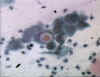
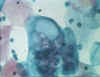 Figure 2 Chlamydial inclusions © Bristol
Biomedical Archive. Used with permission
Figure 2 Chlamydial inclusions © Bristol
Biomedical Archive. Used with permission
 Figure 3 Chlamydial inclusions in an endothelial
cell © Bristol Biomedical Archive. Used with permission
Figure 3 Chlamydial inclusions in an endothelial
cell © Bristol Biomedical Archive. Used with permission
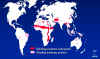 Figure 4 Distribution of trachoma © World Health Organization
Figure 4 Distribution of trachoma © World Health Organization
|
Chlamydia trachomatis
C. trachomatis is
the causative agent of trachoma, urogenital disease, infant pneumonia
and
lymphogranuloma venereum.
Biovars
C. trachomatis has a limited host
range and only infects human epithelial cells (one strain can infect mice).
The species is divided into three
biovars (biological variants): trachoma,
lymphogranuloma venereum and mouse pneumonitis.
Serovars
The human biovars have been further
subdivided in to several serovars (serological variants; equivalent to
serotypes) that differ in
their major outer membrane proteins and which are associated with different
diseases (Table 1)
|
Table
1 |
|
Serovar
|
Disease
|
Distribution
|
|
A B Ba
C
|
Trachoma
|
Asia
and Africa
|
|
D - K
|
Disease
of eye and genitals:
Conjunctivitis
Urethritis
Cervicitis
Respiratory System:
Infant pneumonia
|
World
wide
|
|
LGV1
LGV2 LGV3
|
Lymphogranuloma
venerium
|
Worldwide
|
|
|
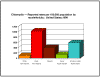 Figure 4A
Figure 4A
Chlamydia - Reported rates per 100,000 population by race/ethnicity: United States,1999
CDC
 Figure 4B
Figure 4B
Chlamydia - Age-and gender-specific rates: United States,1999 CDC
 Figure 5 Results of trachoma-specific
interventions in three countries in the last 30 years
Figure 5 Results of trachoma-specific
interventions in three countries in the last 30 years
© World Health Organization |
Pathogenesis and Immunity
C. trachomatis
infects non-ciliated columnar epithelial cells. The organisms stimulate the
infiltration of polymorphonuclear cells and lymphocytes which leads to
lymphoid follicle formation and fibrotic changes. The clinical
manifestations result from destruction of the cells and the host
inflammatory response. Infection does not stimulate long lasting immunity
and reinfection results in a inflammatory response and subsequent tissue
damage.
Epidemiology
a. C. trachomatis (biovar: trachoma) is found
worldwide primarily in areas of poverty and overcrowding (Figure 4). It is
estimated that 500 million people are infected worldwide and 7 - 9
million people are blind as a consequence. C. trachomatis biovar:
trachoma is endemic in Africa, the Middle East, India and Southeast
Asia. In the United States, Native Americans are most commonly
infected.
Infections occur most commonly in children. The organism can be
transmitted by droplets, hands, contaminated clothing, flies, and by
passage through an infected birth canal.
a. C. trachomatis (biovar: trachoma) is the
most common sexually transmitted bacterial disease in the United States
(4 million new cases each year) and 50 million new cases occur yearly
worldwide. In the United States, the highest infection rates occur in
Native and African Americans (Figure 4A) with a peak incidence in the
late teens/early twenties (Figure 4B).
b. C. trachomatis (biovar: LGV) is a sexually
transmitted disease that occurs sporadically in the United States but is
more prevalent in Africa, Asia and South America. Humans are the only
natural host. Incidence is 300 - 500 cases per year in the United States
with male homosexuals being the major reservoir of the disease.
|
|
|
|
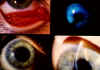 Figure 7 Chlamydial kerato-conjunctivitis © Bristol
Biomedical Archive. Used with permission
Figure 7 Chlamydial kerato-conjunctivitis © Bristol
Biomedical Archive. Used with permission
 Figure 8
Figure 8
Tracomatous SCARRING :
the presence of scarring in the tarsal conjunctiva. Scars are easily visible as white lines, bands, or sheets in the tarsal conjunctiva. They are glistening and fibrous in appearance. Scarring, especially diffuse
fibrosis, may obscure the tarsal blood vessels.
©
World Health Organization
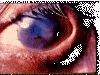 Tracomatous TRICHIASIS : at least one eyelash rubs on the
eyeball © World Health
Organization
Tracomatous TRICHIASIS : at least one eyelash rubs on the
eyeball © World Health
Organization
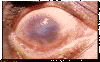 CORNEAL OPACITY : easily visible corneal opacity over the
pupil. The pupil margin is blurred viewed through the opacity. Such corneal opacities cause significant visual impairment (less tah 6/18 or 0.3
vision) © World Health
Organization
CORNEAL OPACITY : easily visible corneal opacity over the
pupil. The pupil margin is blurred viewed through the opacity. Such corneal opacities cause significant visual impairment (less tah 6/18 or 0.3
vision) © World Health
Organization |
Clinical Syndromes
Chronic infection or repeated reinfection
with C. trachomatis (biovar: trachoma) results in inflammation and
follicle formation involving the entire conjunctiva (Figure 7 and 8). Scarring of the
conjunctiva causes turning in of the eyelids and eventual scarring,
ulceration and blood vessel formation in the cornea, resulting in
blindness. The name trachoma comes from 'trakhus' meaning rough
which characterizes the appearance of the conjunctiva. Inflammation in the
tissue also interferes with the flow of tears which is an important
antibacterial defense mechanisms. Thus, secondary bacterial infections
occur.
Inclusion conjunctivitis
is caused by C. trachomatis (biovar: trachoma) associated with
genital infections (serovars D - K). The infection is characterized by a
mucopurulent
discharge, corneal infiltrates and occasional corneal
vascularization. In chronic cases corneal scarring may occur. In neonates
infection results from passage through an infected birth canal and becomes
apparent after 5 - 12 days. Ear infection and rhinitis can accompany the
ocular disease.
Infants infected with C.
trachomatis (biovar: trachoma; serovars: D - K) at birth can develop
pneumonia. The children develop symptoms of wheezing and cough but not
fever. The disease is often preceded by neonatal conjunctivitis.
Infection with the LGV serovars of
C. trachomatis (biovar: LGV) can lead to
oculoglandular conjunctivitis. In addition to the conjunctivitis, patients
also have an associated lymphadenopathy.
In females, the infection is
usually (80%) asymptomatic but symptoms can include cervicitis, urethritis,
and salpingitis. Postpartum fever in infected mothers is common. Premature
delivery and an increased rate of ectopic pregnancy due to
salpingitis can
occur. In the United States, tubal pregnancy is the leading cause of first-trimester, pregnancy-related deaths.
In males, the infection is usually (75%) symptomatic
After a 3 week incubation period patients may develop urethral discharge,
dysuria and
pyuria.
Approximately 35 - 50% of non-gonococcal urethritis is due to C.
trachomatis (biovar: trachoma). Post-gonococcal urethritis also occurs
in men infected with both Neisseria gonorrhoeae and C.
trachomatis. The symptoms of chlamydial infection occur after
treatment for gonorrhea because the incubation time is longer.
Up to 40% of women with untreated (undiagnosed) chlamydia will develop
pelvic inflammatory diseases and about 20% of these women will become
infertile. Many untreated cases (18%) result in chronic pelvic pain.
Women infected with chlamydia have a 3 - 5 fold increased risk of acquiring HIV.
|
|
 Figure 9 Chlamydial urogenital infection in men. After an incubation of
3 weeks, up to 75% of patients show symptoms such as urethral discharge, dysuria and
pyuria
Figure 9 Chlamydial urogenital infection in men. After an incubation of
3 weeks, up to 75% of patients show symptoms such as urethral discharge, dysuria and
pyuria
|
Reiter's syndrome is a triad of
symptoms that include conjunctivitis,
polyarthritis and genital
inflammation. The disease is associated with HLA-B27. Approximately 50 -
65% of patients have an acute C. trachomatis infection at the onset
of arthritis and greater than 80% have serological evidence for C.
trachomatis infection. Other infections (shigellosis or Yersinia
enterocolitica) have also been associated with Reiter's syndrome.
The primary lesion of LGV is a small painless and
inconspicuous vesicular lesion that appears at the site of infection,
often the penis or vagina. The
patient may also experience fever, headache and myalgia. The second stage
of the disease presents as a marked inflammation of the draining lymph
nodes. The enlarged nodes become painful 'buboes' that can eventually
rupture and drain. Fever, headache and myalgia can
accompany the inflammation of the lymph nodes. Proctitis is common in
females; lymphatic drainage from the vagina is perianal. Proctitis in
males results from anal intercourse or from lymphatic spread from the
urethra. The course of the disease is variable but it can lead to genital
ulcers or elephantiasis due to obstruction of the lymphatics.
|
|
|
Laboratory diagnosis
There are several laboratory
tests for diagnosis of C. trachomatis but the sensitivity of the
tests will depend on the nature of the disease, the site of specimen
collection and the quality of the specimen. Since chlamydia are
intracellular parasites, swabs of the involved sites rather than exudate
must be submitted for analysis. It is estimated that as many as 30% of the
specimens submitted for analysis are inappropriate.
Examination of stained cell scrapings for
the presence of inclusion bodies (Figures 2 and 3) has been used for diagnosis but this
method is not as sensitive as other methods.
Culture is the most specific method for
diagnosis of C. trachomatis infections. Specimens are added to
cultures of susceptible cells and the infected cells are examined for the
presence of iodine-staining inclusion bodies.
Iodine stains glycogen in the inclusion bodies. The presence of
iodine-staining inclusion bodies is specific for C. trachomatis
since the inclusion bodies of the other species of chlamydia do not
contain glycogen and stain with iodine.
Direct immunofluorescence and
ELISA kits that detect the group specific LPS or strain-specific outer
membrane proteins are available for diagnosis. Neither is as good as
culture, particularly with samples containing few organisms (e.g.
asymptomatic patients).
Serological tests for diagnosis
are of
limited value in adults, since the tests do not distinguish between
current and past infections. Detection of high titer IgM antibodies is
indicative of a recent infection. Detection of IgM antibodies in neonatal
infection is useful.
Three tests based on
nucleic acid probes are available. These tests are sensitive and specific
and may replace culture as the method of choice.
Treatment and prevention
Tetracyclines, erythromycin
and sulfonamides are used for treatment but they are of limited value in
endemic areas where reinfection is common. Vaccines are of little value and
are not used. Treatment coupled with improved sanitation to prevent
reinfection is the best way to control infection. Safe sexual practices and
prompt treatment of symptomatic patients and their sexual partners can
prevent genital infections.
|
|
|
Chlamydophila psittaci
C. psittaci is the
causative agent of psittacosis (parrot fever). Although the disease was
first transmitted by parrots, the natural reservoir for C. psittaci can be any species of bird. Thus, the disease has also been called ornithosis
from the Greek word for 'bird'.
Pathogenesis
The respiratory tract is the main
portal of entry. Infection is by inhalation of organisms from infected birds
or their droppings. Person-to-person transmission is rare. From the lungs
the organisms enter the blood stream and are transported to the liver and
spleen. The bacteria replicate at these sties where they produce focal areas
of necrosis. Hematogenous seeding of the lungs and other organs then occurs.
A lymphocytic inflammatory response in the alveoli and interstitial spaces
leads to edema, infiltration of macrophages, necrosis and sometimes
hemorrhage. Mucus plugs may develop in the alveoli causing cyanosis and
anoxia.
Epidemiology
Approximately 50 - 100 cases of
psittacosis occur annually in the United States with most infections
occurring in adults. The organism is present in tissues, feces and feathers
of infected birds that are symptomatic or asymptomatic. There may also be
reservoirs in other animals such as cats and cattle. Veterinarians, zoo
keepers, pet shop workers and poultry processing workers are at increased
risk for developing the disease.
|
|
|
|
 Figure 10 Course of psittacosis. In severe disease there is a 5%
mortality rate
Figure 10 Course of psittacosis. In severe disease there is a 5%
mortality rate |
Clinical Syndromes
The illness develops after an
incubation time of 7 - 15 days. Symptoms include fever, chills, headache, a
non-productive cough and a mild
pneumonitis. In uncomplicated cases the
disease subsides by 5-6 weeks after infection. Asymptomatic infections are
common. In complicated cases convulsions, coma and death (5% mortality rate)
can occur. Other complications include carditis, hepatomegaly and
splenomegaly (Figure 10).
Laboratory diagnosis
Laboratory diagnosis is based
on a serological tests. A four-fold rise in titer in paired samples in a
complement fixation test is indicative of infection.
Treatment and prevention
Tetracycline or
erythromycin are the antibiotics of choice. Control of infection in birds by
feeding of antibiotic supplemented food is employed. No vaccine is
available.
|
|
|
Chlamydophila pneumoniae
Chlamydophila pneumoniae is the causative agent of
an atypical pneumonia (walking pneumonia) similar to those caused by Mycoplamsma
pneumoniae and Legionella pneumoniae. In addition it can cause a
pharyngitis, bronchitis, sinusitis and possibly atherosclerosis. The organism
was originally called the TWAR strain from the names of the two original
isolates - Taiwan (TW-183) and an acute respiratory isolate designated AR-39.
It is now considered a separate species of chlamydia.
Pathogenesis
The organism is transmitted person-
to-person by respiratory droplets and causes bronchitis, sinusitis and
pneumonia.
Epidemiology
The infection is common with 200,000 -
300,000 new cases reported annually, mostly in young adults. Although 50% of
people have serological evidence of infection most infections are
asymptomatic or mild. The disease is most common in military bases and
college campuses (crowding). No animal reservoir has been identified.
Potential link to
atherosclerosis: A report
in the Journal of the American College of Cardiology
documented a high incidence of C. pneumoniae in the arteries of
patients with atherosclerosis (79% compared with 4% in the control group).
It is still unproven that the link is causal. However, previous reports
show a high association between presence of antibodies to C. pneumoniae
in serum of patients with atherosclerosis as well as the presence of the
organisms in the coronary and carotid arteries.
Clinical Syndrome
Symptoms include a pharyngitis,
bronchitis, a persistent cough and malaise. More severe infections can
result in pneumonia, usually of a single lobe.
Laboratory diagnosis
Culture is difficult so
serological test are most common. A four-fold rise in titer in paired
samples is diagnostic.
Treatment and prevention
Tetracycline and
erythromycin are the antibiotics of choice. No vaccine is available.
|
|
|
 Return to the Bacteriology Section
of Microbiology and Immunology On-line Return to the Bacteriology Section
of Microbiology and Immunology On-line
This page last changed on
Sunday, March 06, 2016
Page maintained by
Richard Hunt
|















