|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
TURKISH |
VIROLOGY - CHAPTER
TWENTY TWO
VIRAL DISEASES TRANSMITTED
BY VERTEBRATES OR FOR WHICH THE RESERVOIR OR VECTOR IS UNCLEAR
Dr. Margaret Hunt
Professor Emerita
University of South Carolina School of Medicine
|
|
Español |
|
|
Let us know what you think
FEEDBACK |
|
SEARCH |
|
|

 |
|
Logo image © Jeffrey
Nelson, Rush University, Chicago, Illinois and
The MicrobeLibrary |
|
|
VIRAL DISEASES TRANSMITTED
BY RODENTS
|
ARBOVIRUSES - RODENT BORNE |
|
Envelope |
Symmetry |
Genome |
Size*
|
|
Arenaviridae family |
|
|
yes
|
helical
|
single strand RNA
ambisense
segmented
|
 |
|
Bunyaviridae family |
|
|
yes |
helical |
single strand
negative sense
segmented |
 |
|
* Relative size
adapted from White and Fenner , Medical Virology, 1994 |
Note: Rodents can be infected by rabies virus although they are
rarely, if ever, involved in transmission to humans. Rabies is the
subject of a
separate chapter. |
| |
ARENAVIRUS FAMILY
|
ARENAVIRUS FAMILY |
|
VIRUS |
DISEASE |
OCCURRENCE |
| Lassa |
Lassa fever (hemorrhagic
fever) |
Africa |
| Manchupo |
Bolivian hemorrhagic fever |
South America |
| Junin |
Argentine hemorrhagic fever |
South America |
| Whitewater Arroyo |
Whitewater Arroyo
hemorrhagic fever |
Western United States |
| Lymphocytic choriomeningitis
virus (LCMV) |
Lymphocytic choriomeningitis
|
Widespead |
All of the above arenaviruses (and
other arenaviruses causing hemorrhagic fever not listed here) have a rodent
vector. The arenavirus-associated hemorrhagic fevers have a high case-fatality
rate (5 - 35%). The arenaviruses seem to establish persistent infections easily
in certain rodents, which get a viremia and a viruria, and shed virus in urine,
stools and saliva. Humans are thought to acquire infection from contact with
contaminated materials, contaminated food, or aerosolized droppings, nesting
materials, etc. Disease in humans often show the following: dehydration,
hemoconcentration, hemorrhage, shock syndrome, cardiovascular collapse. In 1999-2000, there
were reports of three deaths apparently due to a North
American arenavirus (Whitewater Arroyo). It is not clear if there are other
unrecognized cases of this virus or what the case fatality rate is.
Lymphochoriomeningitis is acquired
from close contact with rodents or rodent contaminated materials or in rodent
breeding facilities. Infections are frequently asymptomatic. Clinical infections
are not usually fatal, but there may be some long-term complications. It is not
associated with hemorrhagic fever, but can cause meningitis, encephalitis,
myelitis.
The incubation period of LCMV
infection is usually between 8 and 13 days. A characteristic biphasic febrile
illness then follows. The initial phase, which may last as long as a week,
typically begins with any or all of the following symptoms: fever, malaise,
anorexia, muscle aches, headache, nausea, and vomiting. Other symptoms that
appear less frequently include sore throat, cough, joint pain, chest pain,
testicular pain, and parotid (salivary gland) pain. Following a few days of
remission, the second phase of the disease occurs, consisting of symptoms of
meningitis (for example, fever, headache, and a stiff neck) or characteristics
of encephalitis (for example, drowsiness, confusion, sensory disturbances,
and/or motor abnormalities, such as paralysis). LCMV has also been known to
cause acute hydrocephalus, which often requires surgical shunting to relieve
increased intracranial pressure. In rare instances, infection results in
myelitis (inflammation of the spinal cord) and presents with symptoms such as
muscle weakness, paralysis, or changes in body sensation. (CDC)
|
|
|
|
|
|
CASE REPORTS
Lymphocytic
choriomeningitis deaths from an Arenavirus infection |
| |
BUNYAVIRUS FAMILY -
HANTAVIRUS GENUS
|
BUNYAVIRUS FAMILY - HANTAVIRUS
GENUS |
|
VIRUS AND VECTOR |
DISEASE |
OCCURRENCE |
| Seoul virus - domestic rat Hantaan virus
- field mouse |
Korean hemorrhagic fever Hemorrhagic
fever with renal syndrome |
Southeast Asia |
| Dobrava virus - field mouse Puumala virus
- bank vole |
Hemorrhagic fever with renal syndrome |
Europe, Asia |
| Sin Nombre virus (SNV) - deer mouse |
Hantavirus pulmonary syndrome (HPS) |
North and South America |
The hantavirus genus differs from other members of Bunyaviridae
in that members are transmitted by rodents (rather than arthropods). Each
hantavirus is only transmitted by a limited number of genera/species of rodent.
Infected rodents can spread virus via saliva, urine (they get a viruria) or
droppings. When fresh urine, droppings or recently contaminated nesting material
is swept up or disturbed, the virus can be aerosolized and inhaled. Some of
these viruses can cause severe disease, but even for these viruses many
infections are sub-clinical, or very mild and never diagnosed.
Associated with
hemorrhagic fever with renal syndrome (HFRS)
Hemorrhagic fever with renal syndrome
(formerly known as
Korean Hemorrhagic Fever)
has a case-fatality rate of about 7%. Other members of the Hantaviruses which
cause HFRS (hemorrhagic fever with renal
syndrome) tend to have a lower fatality rate. Transmission appears to be
via inhalation of, or contact with, rodent urine, droppings or saliva.
HFRS has an incubation time
of about two weeks to a month. First, there is a febrile phase for up to a week.
This is typified by fever but other symptoms include general malaise, nausea,
pain and other flu-like symptoms. This is followed by the
hypotensive phase of a few days (lower platelet count,
tachycardia and hypoxemia). The third phase, the oliguric phase, also lasts
a few days and is characterized by protein in the urine (proteinuria) and renal
failure. Finally, there is the
diuresis
phase of days to weeks typified by excessive urination. The patient then usually
recovers.
Associated with severe
pulmonary syndrome
There is a
group of Hantaviruses found in North and South America that is transmitted by rodents
to humans (by
inhalation or contact with excreta) and which causes Hantavirus Pulmonary Syndrome (HPS)
rather than hemorrhagic fever.
These HPS viruses have a high case fatality rate of
about 36%. The viruses are widely distributed throughout the the Americas but relatively rarely
cause human disease - about 380 known cases so far in the US. Initial symptoms
often include fever, myalgia, nausea, vomiting and a cough; this may progress to
dizziness and shortness of breath as lungs fill with fluid, followed by acute
respiratory distress. There are a several Hantaviruses that have been associated
with this syndrome, one of the best known of the United States HPS-associated
viruses is Sin Nombre virus.
In 2012, there was an outbreak of Hantavirus pulmonary syndrome at Curry Village
in Yosemite National Park in California. The infected persons stayed in tent
cabins at the village which may have been accessible to mice. Of the first eight
patients, three died.
Although Hantavirus came to the public’s attention in 1993 in the Four Corners
region of the southwest of the United States, the virus has been around for much
longer in human disease. It probably broke out in 1993 because there were ideal
conditions for an increase in the deer mouse population thereby causing more
human-mouse contact and spread of the virus. Once the virus was discovered, it
was possible to look for similar viruses in stored tissue samples from patients
who had experienced similar symptoms and the earliest case of HPS was found in a
38-year-old Utah man who contracted a similar disease in 1959.
The disease is not restricted to the Four Corners region since a bridge
inspector in Louisiana who had not been in the Four Corners region developed HPS.
This patient had been infected by a different Hantavirus, called Bayou virus,
carried by the rice rat (Oryzomys palustris). Another Hantavirus that has
caused human infection, called the Black Creek Canal virus, is carried by the
cotton rat (Sigmodon hispidus) while in New York, the Hantavirus New
York-1, appears to be carried by the white-footed mouse (Peromyscus leucopus).
More recently, cases of HPS have been discovered in Argentina, Brazil, Canada,
Chile, Paraguay, and Uruguay, making it a pan-hemispheric disease.
The virus has never been reported to spread by person-to-person contact in the
United States; however, in 1996 there may have been person-to-person Hantavirus
transmission in Argentina.
|
|
|

Annual United States HPS Cases and Case-fatality,
1993-2011
CDC

Deer Mouse Habitat in North America. The deer mouse is found throughout
North America, preferring woodlands, but also appearing in desert areas
CDC

Cotton Rat Habitat in North America. The cotton rat is found in the
southeastern US and down into Central and South America. It inhabits
overgrown areas with shrubs and tall grasses.
CDC

Rice Rat Habitat in North America. The rice rat prefers marshy areas and
is semi-aquatic. It is found in the southeastern US and Central America
CDC

White-footed Mouse Habitat in North America.
The white-footed mouse is found throughout southern New England and the
Mid-Atlantic and southern states, the midwestern and western states, and
Mexico. It prefers wooded and brushy areas, although it will sometime
inhabit more open ground
CDC
|
 CDC scientist collecting specimens from trapped rodents.
CDC/Cheryl Tryon
CDC scientist collecting specimens from trapped rodents.
CDC/Cheryl Tryon
ctt1@cdc.gov |
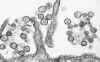 Transmission electron micrograph of a virus that causes Hantavirus pulmonary
syndrome (Sin Nombre virus).
Transmission electron micrograph of a virus that causes Hantavirus pulmonary
syndrome (Sin Nombre virus).
CDC/Cynthia Goldsmith
csg1@cdc.gov
 New World Hanatviruses
New World Hanatviruses
CDC
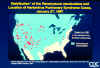 Map of the United States and adjacent areas showing distribution of Peromyscus maniculatus and location of hantavirus pulmonary syndrome cases
Map of the United States and adjacent areas showing distribution of Peromyscus maniculatus and location of hantavirus pulmonary syndrome cases
CDC
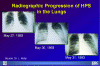 Radiographic Progression of HPS
in the Lungs
Radiographic Progression of HPS
in the Lungs
CDC
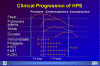 Hantavirus Pulmonary Syndrome Clinical Progression
Hantavirus Pulmonary Syndrome Clinical Progression
CDC
 Hantavirus Pulmonary Syndrome Common Laboratory Findings Hantavirus Pulmonary Syndrome Common Laboratory Findings
CDC
 Histopathology of hanatvirus pulmonary syndrome Other Organs
Histopathology of hanatvirus pulmonary syndrome Other Organs
CDC
|

Hantavirus Pulmonary Syndrome Radiographic Findings
CDC |
 Histopathology of lung in hantavirus pulmonary syndrome. Interstitial pneumonitis and intraalveolar edema.
Histopathology of lung in hantavirus pulmonary syndrome. Interstitial pneumonitis and intraalveolar edema.
CDC/Dr. Sherif R. Zaki sxz1@cdc.gov |
| |
|
SUMMARY
VIRAL DISEASE TRANSMITTED BY RODENTS
|
|
NAME
|
DISEASE
|
OCCURRENCE
|
VECTOR
|
|
Arenavirus
Family
|
|
Lassa fever
|
Hemorrhagic fever
|
Africa
|
rodent
|
|
Bolivian HF*
|
Hemorrhagic fever
|
South America
|
rodent
|
|
Argentine HF*
|
Hemorrhagic fever
|
South America
|
rodent
|
|
Bunyavirus
Family (Hantavirus genus)
|
|
Korean HFRS†
|
Hemorrhagic fever with renal
syndrome
|
SE Asia
|
rodent
|
|
HFRS†
|
Hemorrhagic fever with renal
syndrome
|
Europe and Asia
|
rodent
|
|
Hantavirus pulmonary syndrome ‡
(HPS)
|
Hantavirus pulmonary syndrome
|
N. and S. America
|
rodent

|
|
* Hemorrhagic fever
† Hemorrhagic fever with renal syndrome
‡ Hemorrhagic fever is a feature of all of the above
virus-associated diseases except HPS |
|

Transmission electron micrographs (TEM)
reveal some of the ultrastructural morphology found in the Nipah virus (NiV).
A pleomorphic virus, the image at the top depicts a single long stranded
Nipah virion.
CDC/ Cynthia Goldsmith

Using immunohistochemical (IHC)
technique, this photomicrograph is actually an enlargement of PHIL
12727, from a human central nervous system (CNS) tissue specimen, which
revealed some of the cytoarchitectural histopathologic changes
associated with a Nipah virus infection.
CDC/ Brian W.J. Mahy, BSc, MA, PhD, ScD, DSc |
VIRAL DISEASES TRANSMITTED BY BATS
HENIPAVIRUSES
Hendra
and Nipah viruses (Paramyxoviruses, negative strand RNA viruses)
These two similar paramyxoviruses have their
natural reservoir in and are spread by fruit bats (Flying foxes:
genus Pteropus).
Nipah
virus was first discovered in Malaysia and Singapore in 1999 when it
caused fever and headache after a few days and, in some cases,
respiratory disease. The respiratory symptoms include a very loud
cough. This was followed by confusion, coma and encephalitis.
Sequelae of the infection include convulsions. There were 257
confirmed cases of Nipah virus in the 1999 Malaysia outbreak and
about 40% of patients died. They were all adult males, who were in
close contact with infected pigs. The name Nipah comes from the name
of their village.
Nipah
virus outbreaks have also occurred in India and Bangladesh.
Hendravirus (equine morbillivirus) is named after the Hendra area of
Brisbane, Australia, where it was first discovered in 1994. It
caused respiratory disease (severe flu-like symptoms) and
neurological problems in both horses and three humans. One of the
three humans infected had delayed encephalitis and two died of the
infection. It appears that the humans caught the virus as a result
of direct contact with infected horses.
Diagnosis of both viruses is by ELIZA (to
detect antibodies in the patients) and RT-PCR (to detect the virus
directly). Virus can also be isolated from throat swabs or cerebro-spinal
fluid.
There are no useful drugs to treat infected
patients although ribavirin has been shown to work against the virus
in the laboratory. In 2012, it was shown that a soluble form of the
Hendravirus glycoprotein can protect moneys from challenge with
Nipah virus which may lead to a vaccine against both viruses. It is
planned to use a veterinary vaccine first in Australia, thereby
targeting the spread to humans.
|
 Ebola Virus
Ebola Virus
CDC
 Transmission electron micrograph of Marburg virus. Virions are often seen bent into sixes and hairpin
configurations.
CDC/Dr. Erskine Palmer
Transmission electron micrograph of Marburg virus. Virions are often seen bent into sixes and hairpin
configurations.
CDC/Dr. Erskine Palmer |
VIRAL DISEASES IN WHICH
THE RESERVOIR OR VECTOR IS UNCLEAR
|
VIRUS
DISEASES WITH UNKNOWN RESERVOIR / VECTOR |
|
Envelope
|
Symmetry
|
Genome
|
Size*
|
|
Filoviridae
family |
| yes |
helical |
single strand
negative sense |
 |
|
* Relative size
adapted from White and Fenner , Medical Virology, 1994 |
|
|
|
EBOLA AND MARBURG VIRUSES
Ebola and Marburg viruses
cause hemorrhagic fevers and have a case-fatality rate which can be as high as
60-90% for certain strains of the viruses. These viruses occur in
Africa, but the natural reservoir is unknown. They occasionally infect humans,
but the means by which this occurs is usually not clear.
Patients have severe
hemorrhages and there is a lot of virus present, so stringent barrier nursing
techniques are needed to prevent further spread. There have been a few cases
where humans have been infected by apparently healthy laboratory monkeys.
Ebola virus, which is named
after a river in the Democratic Republic of the Congo, infects humans and other
primates and was first identified in 1976. The virus is a negative strand RNA
filovirus
Disseminated intravascular coagulation (DIC) leading to tissue
ischemia and eventual depletion of clotting factors is a typical feature
of filovirus infections. Currently several anti-clotting agents are
being tested for their effectiveness at preventing the DIC in animal
models.
|
 Negative stain image of an isolate of Marburg virus, showing filamentous particles
Negative stain image of an isolate of Marburg virus, showing filamentous particles
as well as the characteristic "Shepherd's Crook". x100,000.
Image courtesy of Russell Regnery, Ph.D., DVRD, NCID, CDC. |
|
 Return to the Virology section of Microbiology and Immunology On-line
Return to the Virology section of Microbiology and Immunology On-line
This page last changed on
Wednesday, November 23, 2016
Page maintained by
Richard Hunt
|


 CDC scientist collecting specimens from trapped rodents.
CDC/Cheryl Tryon
CDC scientist collecting specimens from trapped rodents.
CDC/Cheryl Tryon 
 Histopathology of lung in hantavirus pulmonary syndrome. Interstitial pneumonitis and intraalveolar edema.
Histopathology of lung in hantavirus pulmonary syndrome. Interstitial pneumonitis and intraalveolar edema.

 Ebola Virus
Ebola Virus Negative stain image of an isolate of Marburg virus, showing filamentous particles
Negative stain image of an isolate of Marburg virus, showing filamentous particles















