In the developing world, shigellosis is far more common and is present in most communities most of the time.

BACTERIOLOGY - CHAPTER ELEVENENTEROBACTERIACEAE, VIBRIO, CAMPYLOBACTER AND
HELICOBACTERDr Alvin Fox
Emeritus Professor
University of South Carolina School of Medicine
FEEDBACK
Logo image © Jeffrey Nelson, Rush University, Chicago, Illinois and The MicrobeLibrary
KEY WORDS
Opportunistic Gastroenteritis
Diarrhea
Dysentery
Urinary tract
infections
Lactose
positive/negative
API strip
Enteropathogenic
E. coli
Enterotoxigenic
E. coli
Heat stable
toxin
Heat labile
toxin
Enteroinvasive
E. coli
Enterohemorrhagic
E. coli
Vero toxin
(Shiga-like)
Hemolysin
Adhesive
pili
Shigella
Bacillary
dysentery
Shiga toxin
Salmonella
typhi
Typhoid
Vi antigen
Salmonella
enteritidis (salmonellosis)
Salmonella
cholerae-suis
Vibrio
cholerae
Cholera
Choleragen
(cholera toxin)
Yersinia
entercolitica
Campylobacter
jejuni
Helicobacter pylori
ENTEROBACTERIACEAE
General
This group of organisms includes several that cause primary infections of the human gastrointestinal tract. Thus, they are referred to as enterics (regardless of whether they cause gut disorders). Bacteria that affect the gastrointestinal tract include certain strains of Escherichia coli and Salmonella, all 4 species of Shigella, and Yersinia entercolitica. The rheumatic disease, Reiter's syndrome (associated with HLA-B27), can result from prior exposure to Salmonella, Shigella, or Yersinia. Other organisms that are not members of the Enterobacteriacae, including Campylobacter and Chlamydia, are also causative agents of Reiter's syndrome. Yersina pestis (the cause of "plague") will be considered separately with other zoonotic organisms.
Members of this family are major causes of opportunistic infection (including septicemia, pneumonia, meningitis and urinary tract infections). Examples of genera that cause opportunistic infections are: Citrobacter, Enterobacter, Escherichia, Hafnia, Morganella, Providencia and Serratia. Selection of antibiotic therapy is complex due to the diversity of organisms.
Some of the organisms additionally cause community-acquired disease in otherwise healthy people. Klebsiella pneumoniae is often involved in respiratory infections. The organism has a prominent capsule aiding pathogenicity . The commonest community acquired ("ascending") urinary tract infection is caused by E. coli. The vast majority of urinary tract infections are ascending, often from fecal contamination. Proteus is another common cause of urinary tract infection; the organism produces a urease that degrades urea producing an alkaline urine.
Isolation and identification of Enterobacteriaceae
Enterobacteriaceae are Gram-negative facultative anerobic rods.
They lack cytochrome oxidase and are referred to as oxidase-negative. They are
often isolated from fecal matter on agar containing lactose and a pH indicator.
Colonies that ferment lactose will produce sufficient acid to cause a color
shift in the indicator (Figure 1). Escherichia coli is a fermenter of lactose, while Shigella,
Salmonella and Yersinia are non-fermenters.
"Non-pathogenic" strains of E. coli (and other lactose-positive
enterics) are often present in normal feces. Since they are difficult to
differentiate from "pathogenic" E. coli, lactose-negative
colonies are often the only ones identified in feces. All Enterobacteriaceae
isolated from other sites (which contain low numbers of bacteria (e.g. urine) or
are normally sterile (e.g. blood)) are identified biochemically, for example
using the API 20E
system. Important serotypes can be differentiated by their O (lipopolysaccharide),
H (flagellar) and K (capsular) antigens. However,
serotyping is generally not
performed in the routine clinical laboratory.
 Figure 1A Reactions in TSI agar slants.
For more information on this figure, please go
here.
© Neal R. Chamberlain, Kirksville College of Osteopathic Medicine, Kirksville, MO
and
The MicrobeLibrary
Figure 1A Reactions in TSI agar slants.
For more information on this figure, please go
here.
© Neal R. Chamberlain, Kirksville College of Osteopathic Medicine, Kirksville, MO
and
The MicrobeLibrary
 Figure 1B Nonlactose fermenter on Hektoen agar which contains
bile salts and acid indicators (bromthymol blue and acid fuchsin). The
gram-positive bacteria are inhibited so the agar is selective for gram-negative
bacteria. The lactose fermenters form orange colonies while the nonfermenters
appear green to blue-green. This is especially helpful in distinguishing
potential pathogens from normal flora in stool specimens. However, it is
difficult to tell the non-fermenters from each other. The organism on this plate
could be Salmonella, Proteus, or Shigella. © Pat Johnson,
Palm Beach Community College, Lake Worth, Florida
and
The MicrobeLibrary
Figure 1B Nonlactose fermenter on Hektoen agar which contains
bile salts and acid indicators (bromthymol blue and acid fuchsin). The
gram-positive bacteria are inhibited so the agar is selective for gram-negative
bacteria. The lactose fermenters form orange colonies while the nonfermenters
appear green to blue-green. This is especially helpful in distinguishing
potential pathogens from normal flora in stool specimens. However, it is
difficult to tell the non-fermenters from each other. The organism on this plate
could be Salmonella, Proteus, or Shigella. © Pat Johnson,
Palm Beach Community College, Lake Worth, Florida
and
The MicrobeLibrary
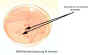 Figure 1B Growth of a nonlactose fermenter
on MacConkey agar which contains bile salts and crystal violet which inhibit the
growth of gram-positive bacteria. The agar also contains lactose and a red dye
that differentiates the lactose fermenters from the non-fermenters. Colonies of
lactose fermenting bacteria are pink to red while the nonfermenters are
colorless or transparent. This agar does not distinguish between the non-lactose
fermenters; this growth could indicate several organisms - Proteus, Salmonella
or Shigella, for example. In a stool specimen, it would be enough
evidence to continue with further identification. © Pat Johnson, Palm Beach
Community College, Lake Worth, Florida
and The MicrobeLibrary
Figure 1B Growth of a nonlactose fermenter
on MacConkey agar which contains bile salts and crystal violet which inhibit the
growth of gram-positive bacteria. The agar also contains lactose and a red dye
that differentiates the lactose fermenters from the non-fermenters. Colonies of
lactose fermenting bacteria are pink to red while the nonfermenters are
colorless or transparent. This agar does not distinguish between the non-lactose
fermenters; this growth could indicate several organisms - Proteus, Salmonella
or Shigella, for example. In a stool specimen, it would be enough
evidence to continue with further identification. © Pat Johnson, Palm Beach
Community College, Lake Worth, Florida
and The MicrobeLibrary
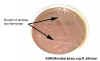 Figure 1C Growth of gram-negative bacteria that cannot
ferment lactose on eosin methylene blue (EMB) agar which contains bile salts and
dyes which inhibit growth of gram-positive bacteria. Growth on EMB agar is a
useful diagnostic tool to distinguish between lactose fermenters and non-fermenters
which will appear colorless. Salmonella and Shigella, both
non-lactose fermenting pathogens, can be distinguished from the more common
intestinal flora which ferment lactose.
© Pat Johnson, Palm Beach Community College, Lake Worth, Florida
and
The MicrobeLibrary
Figure 1C Growth of gram-negative bacteria that cannot
ferment lactose on eosin methylene blue (EMB) agar which contains bile salts and
dyes which inhibit growth of gram-positive bacteria. Growth on EMB agar is a
useful diagnostic tool to distinguish between lactose fermenters and non-fermenters
which will appear colorless. Salmonella and Shigella, both
non-lactose fermenting pathogens, can be distinguished from the more common
intestinal flora which ferment lactose.
© Pat Johnson, Palm Beach Community College, Lake Worth, Florida
and
The MicrobeLibrary
 Figure 2
Figure 2
Colorized scanning electron micrograph. Gram-negative Escherichia coli
bacteria of the strain O157:H7 6836x. CDC
 Figure 3 E. coli (0157:H7) hemorrhagic type. Gram-negative,
enteric, facultatively anaerobic, rod prokaryote. Potentially fatal to
humans, contracted when contaminated meat is cooked inadequately. ©
Dennis Kunkel Microscopy, Inc.
Used with permission
Figure 3 E. coli (0157:H7) hemorrhagic type. Gram-negative,
enteric, facultatively anaerobic, rod prokaryote. Potentially fatal to
humans, contracted when contaminated meat is cooked inadequately. ©
Dennis Kunkel Microscopy, Inc.
Used with permission
Gastroenteritis, diarrhea and dysentery
Escherichia coli
E. coli (figure 3) live in the human gut and are usually harmless but some are pathogenic causing diarrhea and other symptoms as a result of ingestion of contaminated food or water.
At the species level, E. coli and Shigella are indistinguishable. For practical reasons (primarily to avoid confusion), they are not placed in the same genus. Not surprisingly there is a lot of overlap between diseases caused by the two organisms.
1) Enteropathogenic E. coli (EPEC). Certain serotypes are commonly found associated with infant diarrhea. The use of gene probes has confirmed these strains as different from other groups listed below. There is a characteristic morphological lesion with destruction of microvilli without invasion of the organism which suggests adhesion is important. Clinically, one observes:
- fever
- diarrhea
- vomiting
- nausea usually with non-bloody stools
2) Enterotoxigenic E. coli (ETEC) produce diarrhea resembling cholera but much milder in degree. They also cause "travelers' diarrhea". Two types of plasmid-encoded toxins are produced.
- Heat labile toxins which are similar to choleragen (see cholera section below). Adenyl cyclase is activated with production of cyclic AMP and increased secretion of water and ions.
- Heat stable toxins. Guanylate cyclase is activated which inhibits ionic uptake from the gut lumen. Watery diarrhea, fever and nausea result in both cases.
3) Enteroinvasive E. coli (EIEC ) produce a dysentery (indistinguishable clinically from shigellosis, see bacillary dysentery below).
4) Enterohemorrhagic E. coli (EHEC). These are usually serotype O157:H7 (figure 2, 3, 4a). Other kinds of EHEC are sometimes called "non-O157 EHEC". E. coli serogroups O26, O111, and O103 are those that most often cause illness in people in the United States. Most non-O157 EHECs cause less severe disease than O157:H7 but a few can cause more severe symptoms. Very often, non-O157 EHECs are not identified and much less is known about them.
 Figure 4A Transmission electron micrograph of Escherichia coli O157:H7
CDC/Peggy S. Hayes
psh1@cdc.gov
Figure 4A Transmission electron micrograph of Escherichia coli O157:H7
CDC/Peggy S. Hayes
psh1@cdc.gov
 Figure 4B Chronology of E. coli O157:H7 infections, an emerging type of foodborne illness.
CDC
Figure 4B Chronology of E. coli O157:H7 infections, an emerging type of foodborne illness.
CDC
These organisms can produce a hemorrhagic colitis (characterized by bloody and copious diarrhea with few leukocytes in afebrile patients). However, they are taking on increasing importance (figure 4b) with the recognition of outbreaks caused by contaminated hamburger meat. The organisms can disseminate into the bloodstream producing systemic hemolytic-uremic syndrome (hemolytic anemia, thrombocytopenia and kidney failure) which is often fatal. Around 5–10% of those who are diagnosed with EHEC infection develop a potentially life-threatening hemolytic uremic syndrome.
Production of Vero toxin (biochemically similar to Shiga toxin - thus also known as "Shiga-like") is highly associated with this group of organisms. The toxin is encoded by a lysogenic phage. Hemolysins (plasmid-encoded) are also important in pathogenesis.
Since these bacteria make Shiga-like toxins, they are often called “Shiga toxin-producing” E. coli, or STEC for short. They are also called Verocytotoxic E. coli (VTEC); these all refer to the same group of bacteria.
As noted above, there are at least four etiologically distinct diseases. However, in the diagnostic laboratory, the groups are not generally differentiated and treatment is based on symptomatology. Usually, fluid replacement is the primary treatment. Antibiotics are generally not used except in severe disease or disease that has progressed to a systemic stage (e.g. hemolytic-uremia syndrome).
Two major classes of pili are produced by E. coli: mannose-sensitive and mannose-resistant pili. The former bind to mannose containing glyocoproteins and the latter to cerebrosides on the host epithelium, allowing attachment. This aids in colonization by E. coli.
How can EHEC infections be prevented?
 Figure 5. Shigella dysenteriae - Gram-negative, enteric,
facultatively anaerobic, rod prokaryote; causes bacterial dysentery. This
species is most often found in water contaminated with human feces. ©
Dennis Kunkel Microscopy, Inc.
Used with permission
Figure 5. Shigella dysenteriae - Gram-negative, enteric,
facultatively anaerobic, rod prokaryote; causes bacterial dysentery. This
species is most often found in water contaminated with human feces. ©
Dennis Kunkel Microscopy, Inc.
Used with permissionShigella
There are about 14,000 reported cases of shigellosis in the United States each year but because many milder cases are not diagnosed, the actual number of infections is thought to be at least twenty times greater. Shigellosis is particularly common and causes recurrent problems in settings with poor hygiene where epidemics can occur. It is diagnosed more often in summer than winter with children between the ages of 2 to 4 years being the most prone to infection. Often the disease is seen in child care facilities and many cases are the result of the spread of the illness in families with small children. Shigella pass from an infected person to another as they are present in the diarrheal stools. Stools can be infectious while the patient is sick and for up to two weeks after. Most Shigella infections are the result of the bacterium passing from stools or soiled fingers of one person to the mouth of another person. Thus, hygiene is important in containing an outbreak.Shigella (4 species; S. flexneri, S. boydii, S. sonnei, S. dysenteriae (figure 5)) all cause bacillary dysentery or shigellosis, (bloody feces associated with intestinal pain). The organism invades the epithelial lining layer but does not penetrate. Usually within 2 to 3 days, dysentery results from bacteria damaging the epithelial layers lining the intestine, often with release of mucus and blood (found in the feces) and attraction of leukocytes (also found in the feces as "pus"). However, watery diarrhea is frequently observed with no evidence of dysentery. Shiga toxin (chromosomally-encoded), which is neurotoxic, enterotoxic and cytotoxic, plays a role. Its enterotoxicity can make the disease clinically appear as a diarrhea. The toxin inhibits protein synthesis (acting on the 70S ribosome and lysing 28S rRNA). This is primarily a disease of young children occurring by fecal-oral contact. Adults can catch this disease from children, although it can be transmitted by infected adult food handlers who contaminate food. The source in each case is unwashed hands. Man is the only "reservoir".
Managing of dehydration is of primary concern. Indeed, mild diarrhea is often not recognized as shigellosis. Patients with severe dysentery are usually treated with antibiotics (e.g. ampicillin). In contrast to salmonellosis, patients respond to antibiotic therapy and disease duration is diminished.
Incidence of Shigella infections
 Figure 6a. Salmonella - rod prokaryote (dividing); note the flagella. Causes salmonellosis (food poisoning).
(x 20,800) ©
Dennis Kunkel Microscopy, Inc.
Used with permission
Figure 6a. Salmonella - rod prokaryote (dividing); note the flagella. Causes salmonellosis (food poisoning).
(x 20,800) ©
Dennis Kunkel Microscopy, Inc.
Used with permission
 Figure 6b
Figure 6b
Rate of reported Salmonella isolates in US per
100,000 population, 3-month moving average by month and year 1968-2011
CDC
 Figure 7a
Figure 7a
Computer-generated image of five drug-resistant Salmonella serotype
Typhi bacteria based upon scanning electron micrographic image. Note
the presence of numerous thin, short fimbriae emanating from the organisms’
cell wall, imparting a furry appearance to these bacteria, and the multiple
peritrichous flagella.
CDC/Melissa Brower
 Figure 7b
Figure 7b
Rose spots on the chest of a patient with typhoid fever due to the bacterium
Salmonella typhi.
CDC/ Armed Forces Institute of Pathology, Charles N. Farmer
Salmonella
It is estimated that Salmonella cause more than 1.2 million illnesses each year in the United States, resulting in more than 23,000 hospitalizations and 450 deaths. The overall rate of Salmonellosis is falling in the United States although outbreaks periodically occur (figure 6b).
Salmonella infections most often cause vomiting or diarrhea, sometimes severe. In rare cases, Salmonella illness can lead to severe and life-threatening bloodstream infections.
Based on genetic studies, there is a single species of Salmonella (Salmonella enterica) (figure 6a). At the other extreme using appropriate antibodies, more than 2000 antigenic "types" have been recognized. There are, however, only a few types that are commonly associated with characteristic human diseases (most simply referred to as Salmonella enteritidis, Salmonella cholerae-suis and Salmonella typhi).
Salmonellosis
Salmonellosis, the common salmonella infection, is caused by a variety of serotypes (most commonly S. enteritidis) and is transmitted from contaminated food (such as poultry and eggs). It does not have a human reservoir and usually presents as a gastroenteritis (nausea, vomiting and non-bloody stools). The disease is usually self-limiting (2 - 5 days). Like Shigella, these organisms invade the epithelium and do not produce systemic infection. In uncomplicated cases of salmonellosis, which are the vast majority, antibiotic therapy is not useful. S. cholerae-suis (seen much less commonly) causes septicemia after invasion. In this case, antibiotic therapy is required.
Typhoid
The severest form of salmonella infections, "typhoid" (enteric fever), caused by Salmonella typhi (figure 7a), is not often seen in the United States, although it is one of the historical causes of widespread epidemics and still is in the third world. It is estimated that about 5,700 cases occur annually in the United States. Most cases (up to 75%) are acquired while traveling internationally. Typhoid fever affects about 21.5 million persons each year in the developing world.
The organism is transmitted from a human reservoir or in the water supply (if sanitary conditions are poor) or in contaminated food. It initially invades the intestinal epithelium and, during this acute phase, gastrointestinal symptoms are noted. The organisms penetrates (usually within the first week) and passes into the bloodstream where it is disseminated in macrophages. Symptoms of typhoid include a fever up to 103° to 104° F (39° to 40° C). The patient may also feel weak and have stomach pains, headache, and/or loss of appetite. In some cases, patients have a rash of flat, rose-colored spots (figure 7b). Diagnosis of typhoid fever is carried out from stool or blood samples that are tested for the presence of Salmonella typhi.
Typical features of a systemic bacterial infection are seen. The septicemia usually is temporary with the organism finally lodging in the gall bladder. Organisms are shed into the intestine for some weeks. At this time, gastroenteritis (including diarrhea) is noted again. The Vi (capsular) antigen plays a role in the pathogenesis of typhoid. A carrier state is common; thus one person (e.g. a food handler) can cause a lot of spread. Antibiotic therapy is essential. Unfortunately, there is increasing resistance to antibiotics, including fluoroquinolones and, as a result there may be increases in case-fatality rates. Epidemics and high endemic disease rates occur in the Central Asian Republics, the Indian subcontinent, and across Asia and the Pacific Islands.
Most people in the United States are not vaccinated against typhoid but those traveling to a country where typhoid is common, should consider being vaccinated against typhoid. There are two vaccines available: Ty21a, taken orally in a capsule and ViCPS, taken by injection. Both need boosters after a number of years.
 Figure 7c
Figure 7cYersinia enterocolitica - Gram-negative, facultatively anaerobic, rod prokaryote (dividing). This bacterium releases a toxin that causes enteritis with pain resembling appendicitis. © Dennis Kunkel Microscopy, Inc. Used with permission
Yersinia entercolitica
Yersinia entercolitica (figure 7c) infection (Yersiniosis) is a major cause of gastroenteritis (the main clinical symptom) in Scandinavia and elsewhere and is seen in the United States. The organisms are invasive (usually without systemic spread). Typically the infection is characterized by diarrhea, fever and abdominal pain.
Y. enterocolitica infections are seen most often in young children in whom symptoms include:
- fever
- abdominal pain
- diarrhea, which is often bloody.
These symptoms usually develop 4 to 7 days after infection and can last for one to three weeks or more. In older children and adults predominant symptoms include:
- right-sided abdominal pain (this may lead to confusion with appendicitis)
- fever
In a few cases, complications including skin rash, joint pains, or bacteremia can occur.
Uncomplicated cases of diarrhea due to Y. enterocolitica usually resolve without antibiotics but in more severe or complicated infections the use of antibiotics such as aminoglycosides, doxycycline, trimethoprim-sulfamethoxazole, or fluoroquinolones is recommended.Y. enterocolitica can be transmitted by fecal contamination of water or milk by domestic animals or from eating meat products. It is best isolated by "cold" enrichment: when refrigerated this organism survives while others do not.
Yersinia pseudotuberculosis
A similar, but less severe, disease is caused by Y. pseudotuberculosis. The disease is characterized by
- fever
- acute abdominal pain due to mesenteric lymphadenitis that mimics appendicitis.
Secondary symptoms include
- erythema nodosum
- reactive arthritis (Reiter's Syndrome)
Outbreaks have been reported in Canada, Japan, Finland and Russia (among others). Only in a few of the outbreaks has the vector or source of the infection been identified. Unwashed vegetables including iceberg lettuce and carrots have been implicated by epidemiologic investigations as a source of infection; however, the source of the contamination has not been identified.
Vibrio species
Vibrio cholerae
These are Gram-negative rods. They are comma shaped, facultative anaerobes which are oxidase positive. The most important vibrio, Vibrio cholerae (figure 8), is the causative agent of cholera. It has simple nutritional requirements and is readily cultivated. V. cholerae is found in the feces of an infected individual and ends up in the water supply if sewage is untreated. The organism is thus transmitted by drinking contaminated water. The organism survives in fresh water and, like other vibrios, in salt water. Food, after water contamination, is another means of transmission. Thus, it is primarily a disease of the third world. In the United States, it is observed in the occasional international traveler (especially to parts of Africa, Southeast Asia, or Haiti), although it is sometimes seen after ingestion of seafood. Once in the gut, the organism adheres to the epithelium of the intestine without penetration. Adhesion to the microvilli is thus important in pathogenesis. Cholera toxin is then secreted.
Choleragen (cholera toxin) is chromosomally encoded and contains two types of subunit (A and B). The B subunit binds to gangliosides on epithelial cell surfaces allowing internalization of the A subunit. B subunits may provide a hydrophobic channel through which A penetrates. The A subunit catalyses ADP-ribosylation of a regulator complex which in turn activates adenylate cyclase present in the cell membrane of the epithelium of the gut. The overproduction of cyclic AMP in turn stimulates massive secretion of ions and water into the lumen. Dehydration and death (without treatment) result. Thus, fluid replacement is the major component of treatment. Antibiotic therapy (including tetracycline) is additionally used. Vaccination is only partially effective and not generally recommended. It is most commonly used by international travelers.
Cholera is usually an acute, diarrheal illness. It is often mild or asymptomatic, but sometimes it can be more severe. About 5-10% of infected patients develop severe cholera, the early symptoms of which include (CDC):
profuse watery diarrhea, sometimes described as “rice-water stools,”
vomiting
rapid heart rate
loss of skin elasticity
dry mucous membranes
low blood pressure
thirst
muscle cramps
restlessness or irritability
This can lead to:
acute renal failure
severe electrolyte imbalances
coma
If untreated, severe dehydration can rapidly lead to shock and death.
As noted above, diarrhea from people with cholera contains large amounts of infectious bacteria that can contaminate the environment (such as water supplies or food) and infect others, if ingested, thereby spreading the disease. Improved sanitary conditions can prevent the spread of cholera. Washing hands after touching anything that might be contaminated and properly disposing of contaminated items and human waste is essential.
In severe cases of cholera, CDC recommends:
Oral or intravenous hydration is the mainstay of cholera treatment
In conjunction with hydration, treatment with antibiotics is recommended for severely ill patients. It is particularly recommended for patients who are severely or moderately dehydrated and continue to pass a large volume of stool during rehydration treatment. Antibiotic treatment is also recommended for all patients who are hospitalized.
Antibiotic choices should be informed by local antibiotic susceptibility patterns. In most countries, Doxycycline is recommended as first-line treatment for adults, while azithromycin is recommended as first-line treatment for children and pregnant women. During an epidemic or outbreak, antibiotic susceptibility should be monitored through regular testing of sample isolates from various geographic areas.
None of the guidelines recommend antibiotics as prophylaxis for cholera prevention, and all emphasize that antibiotics should be used in conjunction with aggressive hydration.
Education of health care workers, assurance of adequate supplies, and monitoring of practices are all important for appropriate dispensation of antibiotics.
Vibrio parahemolyticus
Vibrio parahemolyticus (figure 9) is the agent that causes vibriosis and is usually transmitted by ingestion of raw seafood (especially oysters). An estimated 4,500 cases of vibriosis occur each year in the United States. The organism lives in brackish saltwater and causes gastrointestinal illness in humans. Vibrio parahemolyticus inhabits coastal waters in the United States and Canada and is present in higher concentrations during summer; it grows best in high concentrations of salt (i.e. it is halophilic). A non-bloody diarrhea is observed but it is not as severe as cholera.
There was an increase in Vibrio parahaemolyticus illnesses associated with consumption of shellfish from several Atlantic coast harvest areas in the United States in 2013 (figure 9).
The symptoms of vibriosis are (CDC):
watery diarrhea
frequently with abdominal cramping
nausea
vomiting
fever and chills
Usually these symptoms occur within 24 hours of ingestion of the bacterium. The disease is usually self-limiting and lasts 3 days. Severe disease is rare and occurs more commonly in persons with weakened immune systems. V. parahaemolyticus can also cause an infection of the skin when an open wound is exposed to warm seawater.
Diagnosis is by isolation of the bacterium from cultures of stool, wound, or blood. For isolation from stool, the use of a selective medium that has thiosulfate, citrate, bile salts, and sucrose (TCBS agar) is recommended by CDC.
Usually treatment is not necessary and there is no evidence that antibiotic treatment decreases the severity or the length of the illness. The patient should be encouraged to drink plenty of water to replace fluids lost through diarrhea. In severe or prolonged illnesses, antibiotics such as tetracycline or ciprofloxicin can be used.
Vibrio vulnificus
Vibrio vulnificus (figure 9d) is another salt loving (halophilic) bacterium that causes cases of vibriosis and again disease is caused by eating contaminated raw seafood or exposure of an open wound to contaminated sea water. In the latter case, infection can lead to skin breakdown and ulceration. Between 1988 and 2006, CDC received reports of more than nine hundred V. vulnificus infections from the Gulf Coast states, where most cases occur.
Infection by V. vulnificus can cause, in non-immunocompromised people:
vomiting
diarrhea
abdominal pain
In immunocompromised people, especially those with chronic liver disease, V. vulnificus can infect the bloodstream (bacteremia), resulting in a severe disease that can be life-threatening. It is characterized by:
fever and chills
decreased blood pressure (septic shock)
blistering skin lesions
About half of V. vulnificus bloodstream infections result in death.
Diagnosis is by stool, wound, or blood cultures.
If V. vulnificus is suspected, treatment should be initiated immediately because antibiotics improve survival with aggressive treatment of the wound site; debridement of infected necrotic tissue or amputation of the infected limb is sometimes necessary. Doxycycline and cephalosporin are indicated or a fluoroquinolone such as levofloxacin, ciprofloxacin or gatifloxacin. Children, in whom doxycycline and fluoroquinolones are contraindicated, can be treated with trimethoprim-sulfamethoxazole plus an aminoglycoside.
ANIMATION
Pathology of Cholera
© Alan House and Mike Hyman, Department of Microbiology, North Carolina State University, Raleigh, N.C.
and
The MicrobeLibrary
 Figure 8a
Figure 8aVibrio cholerae. Leifson flagella stain (digitally colorized). CDC/Dr. William A. Clark
 Figure 8b
Figure 8b
Vibrio cholerae - Gram-negative, facultatively
anaerobic, curved (vibrio-shaped), rod prokaryote; causes Asiatic cholera. ©
Dennis Kunkel Microscopy, Inc.
Used with permission
 Figure 9a
Figure 9aVibrio parahaemolyticus - halophilic, facultative anerobic, rod bacterium that causes a food-borne illness known as seafood poisoning. Usually transmitted through eating raw or undercooked seafood such as oysters. Less commonly, this organism can cause an infection in the skin when an open wound is exposed to warm seawater. © Dennis Kunkel Microscopy, Inc. Used with permission
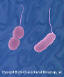 Figure 9b
Figure 9b
Vibrio parahaemolyticus - halophilic, facultative anerobic,
rod bacterium that causes a food-borne illness known as seafood poisoning.
Usually transmitted through eating raw or undercooked seafood such as
oysters. Less commonly, this organism can cause an infection in the skin
when an open wound is exposed to warm seawater.
©
Dennis Kunkel Microscopy, Inc.
Used with permission
 Figure 9c
Figure 9c
Increase in Vibrio parahaemolyticus illnesses associated with
consumption of shellfish from several Atlantic coast harvest areas, United
States, 2013
 Figure 9d
Figure 9d
Scanning electron micrograph of Vibrio vulnificus bacteria; Mag.
13184x CDC
 Figure 10a. Campylobacter fetus. Leifson flagella stain (digitally colorized).
CDC/Dr. William A. Clark
Figure 10a. Campylobacter fetus. Leifson flagella stain (digitally colorized).
CDC/Dr. William A. Clark
 Figure 10b
Figure 10b
Campylobacter jejuni is an enteric, curved-rod
prokaryote (bacterium). It is the bacterium that causes campylobacteriosis,
one of the most common bacterial causes of diarrheal illness in the United
States. It is a relatively fragile bacterium that is easily killed by cold
or hot temperatures. Birds are carriers due to their body temperature
being just right to host the bacteria. Improper handling of raw poultry or
undercooked fowl is usually the source of infection in humans. ©
Dennis Kunkel Microscopy, Inc.
Used with permission
Campylobacter and Helicobacter
These two groups of Gram-negative organisms are both curved or spiral shaped and are genetically related.
Campylobacter jejuni
Campylobacteriosis is one of the commonest bacterial disease causing diarrhea in the United States. There are approximately 14 cases each year per 100,000 population. However, many cases are not diagnosed and it is estimated that there are over 1.3 million cases annually. Infections occur much more frequently in the summer than in winter and the disease occurs in infants and young adults more often than in older people. It is more often seen in males than females. Campylobacteriosis is rarely fatal but there are approximately 76 deaths in the United States among persons with Campylobacter infections each year.
The most common of the Campylobacter (figure 10) causing human disease are C. jejuni. The organism infects the intestinal tract of several animal species (including cattle and sheep) and is a major cause of cause of abortions. It is transmitted to man in milk and meat products. Watery diarrhea predominates but dysentery is common. The organism is invasive but generally less so than Shigella. Malaise, fever and abdominal pain are other disease features. Bacteremia is observed in a small minority of cases.
Campylobacter infection is diagnosed when a culture of a stool specimen yields the bacterium. The organism is microaerophilic and grows best at 42oC. It is frequently isolated under these conditions using selective media . It can be treated with antibiotics but is usually a self-limiting disease. Patients should drink extra fluids as long as the diarrhea lasts. Antibiotics are only used to treat patients with severe disease or those at high risk for severe disease. These include patients with immune systems severely weakened from medications or other illnesses. Azithromycin and fluoroquinolones (e.g., ciprofloxacin) are commonly used for treatment of these infections, but resistance to fluoroquinolones is common.
Campylobacteriosis can sometimes have long-term sequelae. These include:
- arthritis.
- Guillain-Barré syndrome. This is a rare disease that affects the nerves of the body beginning several weeks after the diarrheal illness and results from an attack on body's nervous system by the immune system and can result in temporary paralysis for several weeks. It requires intensive medical care. It is estimated that about one in every 1,000 Campylobacter illnesses leads to Guillain-Barré syndrome. As many as 40% of Guillain-Barré syndrome cases in the United States may result from campylobacteriosis.
Helicobacter pylori
Helicobacter pylori (figure 11) has been accepted in the last few years as the major cause of stomach ulcers. The organism chronically lives in and on the stomach mucosa of man. Culture is the preferred method of diagnosis but may miss a number of cases. The organism characteristically produces a urease which generates ammonia and carbon dioxide. This aids in detecting and identifying the isolated organism. Urease is produced in such large amounts that it can be directly detected in mucosa sampled after endoscopy. Alternatively, 13C or 14C labeled CO2 is detected in the breath after feeding labeled urea. Production of ammonia is a factor in pathogenesis (in locally neutralizing stomach acid). Antibiotic therapy eliminates the organism, peptic ulcers heal and relapses are generally avoided.
Conclusion
Sanitary measures protect the water
supply, avoiding contamination with sewage. This is the primary reason that
epidemics with life-threatening pathogens (e.g cholera and typhoid) are rarely
seen in western countries but are commonly seen in the third world. Other less severe
diseases (e.g. salmonellosis, EHEC) are still common from eating contaminated
animal products, which has been less well controlled. Shigella, which has
a human host, would be even more difficult to eradicate. Vaccination is rarely
used and, indeed, is an expensive way to go compared to sewage treatment. In
severe diarrhea, fluid replacement is essential. Antibiotic therapy is used in
severe local infection and always in systemic disease.
 Figure 10c
Figure 10cCampylobacter jejuni - Gram-negative, enteric, curved (vibrio-shaped), rod prokaryote. Found in the gastrointestinal tract of humans and animals, it can travel to the oral cavity and genitourinary tract. Causes gastroenteritis, especially in infants. © Dennis Kunkel Microscopy, Inc. Used with permission
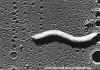
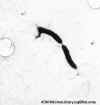 Figure 11a
Figure 11aHelicobacter pylori electron micrographs; fastidious microaerophile; typical helical shape shown in EM; causative agent of chronic gastritis, peptic ulcers and gastric cancer. Image can be used to describe the helical morphology of the organism. Average size: 1micron by 2-5 microns. Organism is
in log phase of growth. © Cindy R. DeLoney, Loyola University of Chicago, Chicago, Illinois and The MicrobeLibrary
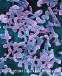 Figure 11b
Figure 11b
Helicobacter pylori - Gram-negative, spiral to pleomorphic, spiral rod
prokaryote. It can move by means of tiny flagella at the end of the cell.
There are many strains of H. pylori which are distinguished by the
human disease with which they cause. H. pylori infection is the main
cause of chronic superficial gastritis and it is associated with both
gastric and duodenal ulcers. It lives in the interface between the surface
of gastric epithelial cells (the lining of the stomach). It often clusters
at the junctions of epithelial cells.
©
Dennis Kunkel Microscopy, Inc.
Used with permission
 Figure 11c
Figure 11cHelicobacter pylori - Gram-negative, spiral to pleomorphic, spiral rod prokaryote. © Dennis Kunkel Microscopy, Inc. Used with permission
![]() Return to the Bacteriology
Section
of Microbiology and Immunology On-line
Return to the Bacteriology
Section
of Microbiology and Immunology On-line
This page last changed on
Monday, February 29, 2016
Page maintained by
Richard Hunt
