|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
|
IMMUNOLOGY -
CHAPTER NINETEEN
IMMUNODEFICIENCY
Abdul
Ghaffar, Ph.D.
Emertius Professor of Pathology, Microbiology and Immunology
University of South Carolina
|
|
FRANCAIS |
|
TURKISH |
Let us know what you think
FEEDBACK |
|
SEARCH |
| |
|
|
 |
|
Logo image © Jeffrey
Nelson, Rush University, Chicago, Illinois and
The MicrobeLibrary |
|
|
|
TEACHING
OBJECTIVES
Know the
primary and secondary immunodeficiencies
Know
immunodeficiencies in AIDS and other conditions
|
IMMUNODEFICIENCY
Immunodeficiency is the
failure of the immune system to protect against disease or malignancy.
Primary Immunodeficiency is caused by genetic or developmental defects
in the immune system. These defects are present at birth but may show up
later on in life.
Secondary or acquired immunodeficiency is the loss of
immune function as a result of exposure to disease agents, environmental
factors, immunosuppression, or aging. |
|
Know the major
primary immunodeficiencies and their features
Understand the
relationship between site of lesion and resulting immunodeficiency
Know the
diagnostic tests for different immunodeficiencies |
PRIMARY
IMMUNODEFICIENCIES
Primary immunodeficiencies are
inherited defects of the immune system (figure 1). These defects may be in the
specific or non-specific immune mechanisms. They are classified on the
basis of the site of lesion in the developmental or differentiation
pathway of the immune system.
Individuals with
immunodeficiencies are susceptible to a variety of infections and the type
of infection depends on the nature of immunodeficiency (Table 1).
| Table
1. Characteristic infections of the primary immunodeficiencies |
|
Component |
Primary pathogen |
Primary site |
Clinical example |
|
T-cells |
Intracellular, bacteria
viruses, protozoa, fungi, |
Non-specific |
SCID, DiGeorge |
|
B-cells |
Pneumococcus,
Streptococcus, Haemophilus
|
Lung, skin, CNS
|
IgG, IgM deficiency
|
|
Enteric
bacteria and viruses |
GI,
nasal, eye |
IgA
deficiency |
|
Phagocytes |
Staphylococcus,
Klebsiella Pseudomonas |
Lung, skin, regional
lymph node |
chronic granulomatous
disease (CGD) |
|
Complement |
Neisseria, Haemophilus,
Pneumococcus, Streptococcus |
CNS,
lung,
skin
|
C3, Factors I and H,
late C components |
|
|
|
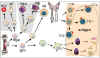 Figure 1
Figure 1
Developmental defects in primary immunodeficiencies |
| |
Specific immune system
There are variety of
immunodeficiencies which result from defects in stem cell differentiation
and may involve T-cells, B-cells, and/or immunoglobulins of different
classes and subclasses (Table 2).
A defect in the early hematopoiesis
which involves stem cells results in reticular
dysgenesis that leads to
general immune defects and subsequent susceptibility to infections. This
condition is often fatal but very rare. It can be treated successfully by
bone marrow transplantation.
Lymphoid lineage
immunodeficiency
If the lymphoid progenitor cells are defective, then both the T and B
cell lineages are affected and result in the severe combined
immunodeficiency (SCID). Infants suffer from recurrent infections
especially by opportunistic microrganisms (bacterial, viral, mycotic and protozoan
infections).
In about 50% of SCID patients,
the immunodeficiency is x-linked whereas in the other half the deficiency
is autosomal. Both are characterized by an absence of T cell and B
cell immunity and absence (or very low numbers) of circulating T and B
lymphocytes. Thymic shadows are absent on X-rays.
The x-linked severe SCID
is due to a defect in the gamma-chain of IL-2 also shared by IL-4, -7,
-11 and 15, all of which are involved in lymphocyte proliferation and/or
differentiation. The autosomal SCIDs arise primarily from defects in
adenosine deaminase (ADA) or purine nucleoside phosphorylase (PNP)
genes which results is accumulation of dATP or dGTP, respectively, and
cause toxicity to lymphoid stem cells.
Other genetic defects leading to SCID include those for RAG1, RAG2 and IL-7-alpha. If suspected of SCID, the patient must not receive live vaccine, as it will result in
progressing disease.
Diagnosis is based on
enumeration of T and B cells and immunoglobulin measurement. Severe
combined immunodeficiency can be treated with a bone marrow transplant (see
MHC and transplantation). Recently, autosomal SCID patients with ADA
deficiency have been treated with a retroviral vector transfected with the
gene with some success.
SCID includes several
disorders
Recombinase activating
genes
Patients having both T and B
cell deficiency lack recombinase activating genes (RAG1 and 2) that are
responsible for the T cell receptor and immunoglobulin gene rearrangements. These
patients are athymic and are diagnosed by examining the T cell receptor
(TCR) gene rearrangement. Defects in B cells are not observed in early
infant life because of passive antibodies obtained from the mother. NK cells are
normal in these patients. This is an
autosomal recessive trait.
CD3 chain
In some SCID patients, T cells
may be present but functionally defective because of deficiency in
signaling mediated by the CD3 chain that is associated with the TCR.
Interleukin-2 receptor
Interleukin-2 receptor common
gamma chain (IL-2Rγc) may be lacking in patients thereby preventing
signaling by IL-2 and other cytokines which act as growth factors. This
leads to a defect in the proliferation of T cells, B cells and NK cells.
This is an
autosomal recessive trait.
Adenosine deaminase
Adenosine deaminase (ADA) is
an enzyme responsible for converting adenosine to inosine. ADA deficiency leads to
accumulation of adenosine which results in the production of toxic
metabolites that interfere with DNA synthesis. The
patients have defects in T, B and NK cells.
SCIDs are autosomal recessive
traits and can be treated by gene therapy or stem cell transplantation.
| Table
2. Summary of T cell and B cell immunodeficiency diseases (ID) |
|
Disease |
T-cells |
B-cells
No
|
Immunoglobulins |
Inheritance |
|
No. |
Fx |
IgM |
IgG |
IgA |
|
Reticular dysgenesis
|
A |
A |
A |
A |
A |
A |
u |
| CID
(autosomal) |
A/L |
A/L |
A/L |
A/L |
A/L |
A/L |
a |
| SCID
(x-linked) |
A/L |
A/L |
A/L |
A/L |
A/L |
A/L |
x |
| DiGeorge's
syndrome |
A/L |
A/L |
N/V |
N/V |
N/V |
N/V |
a/x |
|
Ataxia
telangiectasia |
L |
L |
L |
N/V |
L/V |
L |
a
|
|
Wiskott-Aldrich
|
?V |
L |
L/V |
L |
N |
H |
x |
| also
high IgE |
|
X-linked
hypo-gamma- globulinemia |
N |
N |
L |
L |
L |
L |
x |
|
Selective
IgA immunodeficiency |
N |
N |
N |
N |
L/V |
L |
a/x |
|
Hyper-IgM
hypo-gamma- globulinemia |
N |
N |
N |
H |
L |
L |
x |
|
Transient
hypo-gamma- globulinemia |
N |
N |
N |
N |
L |
L |
a? |
|
Common
variable hypo-gamma- globulinemia (teens-adult) |
N |
N |
N |
N |
L |
L |
none |
| A:
absent; a: autosomal; H: high; L: low; N: normal; U; unknown; V:
variable; x: x-linked |
|
|
|
Disorders of T
cells
T cell disorders affect both cell-mediated and
humoral immunity making the patient susceptible to viral, protozoal and
fungal infections. Viral infections such as those by cytomegalovirus and
attenuated measles
in the vaccine can be fatal in these patients.
DiGeorge's Syndrome (Deletion 22 Syndrome)
This
the most clearly defined T-cell immunodeficiency and is also known as
congenital thymic aplasia/hypoplasia, or immunodeficiency with
hypoparathyroidism. The syndrome is associated with hypoparathyroidism,
congenital heart disease, low set notched ears and fish shaped mouth.
These defects results from abnormal development of the fetus (3rd and 4th
pharyngeal pouch) during the 6th
to 10th
week of gestation when parathyroid, thymus, lips, ears and aortic arch are
being formed. No genetic predisposition is clear and not all DiGeorge
syndrome babies have thymic aplasia. A thymic graft taken from an early
fetus (13 - 14 weeks of gestation) can be used for treatment. Older grafts
may result in GVH reaction. In severely immunodeficient DiGeorge patients,
live vaccines may cause progressive infections.
DiGeorge syndrome is autosomal dominant (figure
2) and is
caused by a deletion in chromosome 22 (figure 3). The deletions are of variable size
but size does not correlate with severity of disease. In about 6% of
cases, the chromosome 22 microdeletion is inherited but most cases result
from de novo deletion which may be caused by environmental factors.
Patients may be treated with a thymic graft.
|
|
CASE
PRESENTATION
Pediatric
Pathology
DiGeorge Syndrome
A 24-day-old Term Infant with Seizures
(Department of Pathology, University of Pittsburgh) |
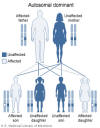 Figure 2
Figure 2
In DiGeorge's syndrome, 22q11.2 deletion is inherited in an autosomal
dominant pattern. National Library of Medicine - NIH
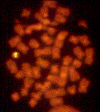 Figure 3
Figure 3
Deletion of genes in DiGeorge syndrome can be visualized by a fluorescent
signal on only one of the two copies of chromosome 22
David Ian Wilson, University of Newcastle on Tyne - NIH |
T cell deficiencies with
variable degrees of B cell deficiency
Ataxia-telangiectasia
Ataxia-telangiectasia
is a deficiency of T cells associated with a lack of coordination of
movement (ataxis) and dilation of small blood vessels of the facial area (telangiectasis).
T-cells and their functions are reduced to various degrees. B cell numbers
and IgM concentrations are normal to low. IgG is often reduced and IgA
is considerably reduced (in 70% of the cases). There is a high
incidence of malignancy, particularly leukemias, in these patients. The
defects arise from a breakage in chromosome 14 at the site of TCR and
immuinoglobulin
heavy chain genes.
Wiskott-Aldrich
syndrome
Wiskott-Aldrich syndrome syndrome is associated with normal T cell numbers with reduced
functions, which get progressively worse. IgM concentrations are
reduced but IgG levels are normal. Both IgA and IgE levels are elevated.
Boys with this syndrome develop severe eczema, petechia (due to platelet
defect and thrombocytopenia). They respond poorly to polysaccharide
antigens and are prone to
pyogenic infection. Wiskott-Aldrich
syndrome is an X-linked disorder (figure 4) due to defect in a cytoskeletal
glycoprotein, CD43.
MHC deficiency (Bare leukocyte
syndrome)
A number of cases of immunodeficiency have
been described in which there is a defect in the MHC class II
transactivator (CIITA) protein gene, which results in a lack of class II MHC molecules on their APC. Since the positive selection of CD4 cells in
the thymus depends on the presence of these MHC molecules, these patients
have fewer CD4 cells and are infection prone. There are also individuals
who have a defect in their transport associated protein (TAP) gene and
hence do not express the class I MHC molecules and consequently are
deficient in CD8+ T cells.
|
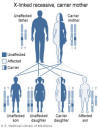 Figure 4
Figure 4
Wiskott-Aldrich syndrome is an X-linked disorder
National Library of Medicine - NIH |
Disorders of B lymphocytes
There are a number of diseases
in which T cell numbers and functions are normal: B cell numbers may be
low or normal but immunoglobulin levels are low.
X-linked infantile hypogammaglobulinemia
X-linked
hypogammaglobulinemia, also referred to as Bruton's hypoglobulinemia or
agammaglobulinemia, is the most severe hypogammaglobulinemia in which B
cell numbers and all immunoglobulin levels are very low. The patients have
failure of B-cell maturation associated with a defective B cell
tyrosine kinase (btk) gene. Thus, B cells exist as pre-B cells with
H chains but not L chains rearranged. Diagnosis is based on enumeration
of B cells and immunoglobulin measurement. Patients have no
immunoglobulins and suffer from recurrent bacterial infections.
Transient hypogammaglobulinemia
Children, at birth, have IgG levels comparable to that of the mother.
Because the
half life of IgG is about 30 days, its level gradually declines, but by three
months of age normal infants begin to synthesize their own IgG. In some
infants, however, IgG synthesis may not begin until they are 2 to 3 years
old. This delay has been attributed to poor T cell help. This results in a
transient deficiency of IgG which can be treated with gamma-globulin.
Common variable
hypogammaglobulinemia (Late onset hypogammaglobulinemia)
These
individuals have deficiencies of IgG and IgA in the 2nd or 3rd
decade of their life because B cells fail to differentiate into
plasma cells. These patients are susceptible to a variety of pyogenic bacteria
and intestinal protozoa. They should be treated with specially prepared
gamma-globulin for intravenous use.
IgA deficiency
IgA
deficiency is the commonest of all immunodeficiencies (1/700 of all
Caucasians) and results from a defect in class switching. About 20% of individuals with IgA deficiency also have low
IgG. IgA-deficient patients are very susceptible to gastrointestinal, eye
and nasopharyngeal infections. Patients with IgA deficiency have a high
incidence of autoimmune diseases (particularly immune complex type) and
lymphoid malignancies. Anti-IgA antibodies (IgG) are detected in 30 to 40
percent of patients who should not be treated with γ-globulins.
Laboratory diagnosis is based on IgA measurement.
Selective IgG deficiency
Deficiencies
of different IgG subclasses have been found. These patients are
susceptible to pyogenic infections.
X-linked Hyper-IgM immunodeficiency
Individuals
with this type of immunodeficiency have low IgA and IgG concentrations
with abnormally high levels of IgM. These patients cannot make a switch
from IgM to other classes which is attributed to a defect in CD40L on
their CD4 cells. They are very susceptible to pyogenic infection and
should be treated with intravenous gamma-globulins.
|
 Figure 5
Figure 5
Poor intracellular killing of bacteria in chronic
granulomatous disease |
Non-specific immune system
- DEFECTS IN THE MYELOID LINEAGE
Primary immunodeficiencies of
the non-specific immune system include defects in phagocytic and NK cells
and the complement system.
Congenital Agranulomatosis
Patients have a decrease in the neutrophil count. This is due to a
defect in the myeloid progenitor cell differentiation into neutrophils.
These patients are treated with granulocyte-macrophage colony
stimulating factor (GM-CSF) or G-CSF.
Defects of the phagocytic
system
Defects of phagocytic cells
(numbers and/or functions) can lead to increased susceptibility to a
variety of infections.
Cyclic neutropenia
This
is marked by low numbers of circulating neutrophil approximately every
three weeks. The neutropenia lasts about a week during which the patients
are susceptible to infection. The defect appears to be due to poor
regulation of neutrophil production.
Chronic granulomatous disease (CGD)
CGD is characterized by marked
lymphadenopathy, hepato- splenomegaly and chronic draining lymph nodes.
Leukocytes have poor intracellular killing (figure 5) and low respiratory burst. In
majority of these patients, the deficiency is due to a defect in NADPH
oxidase (cytochrome b558 : gp91phox, or
rarely gp22phox) or other cofactor proteins (gp47phox,
gp67phox) that participate in phagocytic respiratory
burst. These patients can be diagnosed on the basis of poor Nitroblue
tetrazolium (NBT) reduction which is a measure of respiratory burst.
Interferon-gamma therapy has been successful.
|
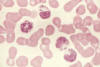 Figure 6
Figure 6
This slide is from a
patient with Chediak-Higashi Syndrome. Extremely large granules are seen in
the cytoplasm of granulocytes. They result from abnormal fusion of granules
during their formation. The abnormal granules are found in many other cell
types throughout the body
National Cancer Inst |
Leukocyte Adhesion Deficiency
In this disease,
T cells and macrophages lack the complement receptor CR3 due to a defect in CD11 or
CD18 peptides and consequently they cannot respond to C3b opsonin.
Alternatively there may a defect in integrin molecules, LFA-1 or mac-1
arising from defective CD11a or CD11b peptides, respectively. These
molecules are involved in
diapedesis and hence defective neutrophils
cannot respond effectively to chemotactic signals. Treatment is with
bone marrow (devoid of T cells and MHC-matched) transplantation or gene
therapy.
Chediak-Higashi syndrome
Chediak-Higashi syndrome is marked by
reduced (slower rate) intracellular killing and chemotactic movement
accompanied by inability of phagosome and lysosome fusion and proteinase
deficiency. Giant lysosomes (intracellular granules) are often seen
(figure 6). The respiratory burst is normal. Accompanying NK cell defects and
platelet and neurological disorders are noted.
|
| |
Disorders of complement system
Complement abnormalities also
lead to increased susceptibility to infections. There are genetic
deficiencies of various components of complement system, the most serious
of which is the C3 deficiency
which may arise from low C3 synthesis or deficiency in factor I or factor
H.
|
| |
SECONDARY (ACQUIRED) IMMUNODEFICIENCIES
Immunodeficiencies associated
with infections
Bacterial, viral, protozoan,
helminthic and fungal infections may lead to B cell, T cell, PMN and
macrophage deficiencies. Most prominent among these is acquired
immunodeficiency syndrome (AIDS). Secondary immunodeficiencies are also
seen in malignancies.
Immunologic abnormalities in the
AIDS
All acquired immunodeficiencies have been outdone by AIDS that is caused
by Human Immunodeficiency Virus (HIV)-1. This virus was first discovered
in 1981 and the patients exhibited fungal infections with opportunistic
organisms such as
Pneumocystis carinii and in other
cases, with a skin tumor known as Kaposi's sarcoma. There are two major
types of HIV: HIV-1 and 2, the former being the strain frequently found
in North America. HIV is spread through sexual intercourse, infected
blood and body fluids as well as from mother to offspring. HIV is a retrovirus with RNA that is reverse transcribed
to DNA by reverse transciptase (RT) following entry into the cell. The
DNA is integrated into the cell genome as a provirus that is replicated
along with the cell. HIV-1 does not replicate in most other animals but
infects chimpanzees although it does not induce AIDS in them. Severe
combined immunodeficient mice (SCID) reconstituted with human
lymphocytes can be infected with HIV-1. The HIV-1 virion consists of a viral
envelope made up of the outer lipid bilayer of the host cell in which
are embedded glycoproteins composed of the transmembrane gp41 along with
the associated gp120. The gp120 binds the CD4 expressed on host cells.
Within the viral envelope is the viral core or nucleocapsid consisting
of a layer of matrix protein composed of p17 and an inner capsid made up
of p24. The viral genome consists of two single stranded RNA molecules
associated with two RT molecules as well as other enzymes including a
protease and an
integrase.
Replication cycle and
targets of therapy
The virus attaches to the CD4 molecule on Th cells, monocytes and
dendritic cells through the gp120 of HIV. For HIV infection, a
co-receptor is required. The co-receptor is a chemokine receptor
such as CXCR4 or CCR5. CCR5, expressed predominantly on macrophages,
and CXCR4 on CD4+ T cells serve as coreceptors for HIV infection.
After the fusion of HIV envelope and the host membrane, the
nucleocapsid enters the cell. The RT synthesizes viral DNA which is
transported to the nucleus where it integrates with the cell DNA in
the form of a
provirus.
The provirus can remain latent until the cell
is activated when the provirus also undergoes transcription. Virions,
consisting of the transcribed viral RNA and proteins, are produced.
These bud out of the host cell membrane from where they acquire the
envelope. Thus, therapeutic agents have been developed that target
viral entry and fusion, as well as serve as RT, protease and
integrase inhibitors.
Highly active anti-retroviral therapy is a
cocktail of 3 or more such agents.
Immunological Changes
The virus replicates rapidly and within about two weeks the
patient may develop fever. The viral load in the blood increases
significantly and peaks in two months, after which there is a sudden
decline because of the latent virus found in germinal centers of the
lymph nodes. CTL develop very early whereas antibodies can be
detected between 3 - 8 weeks. The CTL killing of Th cells around 4 - 8
weeks leads to a decrease in CD4+ T cells. When the CD4+ T cell count
decreases below 200 per cubic mm, full blown AIDS develops.
Immunotherapy
There are several barriers to development of an effective HIV
vaccine.
-
Attenuated vaccine may
induce the disease
-
CD4+ T cells may be
destroyed by the vaccine
-
Antigenic variation of
HIV
-
Low immunogenicity of
the virus by downregulation of MHC molecules
-
Lack of animal models
-
Lack of in vitro tests
The following reagents
have been considered in developing vaccines
-
Immunization with
deletion mutants to reduce pathogenicity
-
Vaccination with
recombinant proteins
-
Gene encoding proteins
introduced into virus vectors may be used for vaccination
-
Chemokines that
compete for the co-receptors
-
IL-2 to boost the Th
cells.
For more on HIV and AIDS
go here
Immunodeficiencies associated
with aging
These include a
progressive decrease in thymic cortex, hypo-cellularity of and reduction
in the size of thymus, a decrease in suppressor cell function and hence an
increase in auto-reactivity, a decrease in CD4 cells functions. By
contrast B cells functions may be somewhat elevated.
Immunodeficiencies associated
with malignancies and other diseases
B cell deficiencies have been
noted in multiple myeloma,
Waldenstrom's macroglobulinemia,
chronic
lymphocytic leukemia and well differentiated lymphomas. Hodgkin's disease
and advanced solid tumors are associated with impaired T-cell functions.
Most chemotherapeutic agents used for treatment of malignancies are also
immunosuppressive.
Other conditions in which
secondary immunodeficiencies occur are sickle cell anemia, diabetes
mellitus, protein calorie malnutrition, burns, alcoholic cirrhosis,
rheumatoid arthritis, renal malfunction, etc.
|
|
|
 Return to the Immunology Section of Microbiology and Immunology On-line
Return to the Immunology Section of Microbiology and Immunology On-line
This page last changed on
Saturday, April 02, 2016
Page maintained by
Richard Hunt
|

 Figure 1
Figure 1 Figure 2
Figure 2 Figure 4
Figure 4 Figure 5
Figure 5 Figure 6
Figure 6
