INFECTIOUS DISEASECHAPTER THREE
LOWER RESPIRATORY TRACT INFECTIONS
Dr Charles Bryan
Emeritus Professor
University of South Carolina School of Medicine
Let us know what you think
FEEDBACK
TEST YOUR KNOWLEDGE
Barking cough
Non-productive
cough
Productive cough
Walking pneumonia
Rusty sputum
Respiratory
alkalosis
Interstitial
infiltrate
|
Text links in maroon go to a dictionary definition Text links in blue go to basic science chapters in this book |
 Photomicrograph of Bordetella (Haemophilus) pertussis bacteria using
Gram stain technique. CDC
Photomicrograph of Bordetella (Haemophilus) pertussis bacteria using
Gram stain technique. CDC
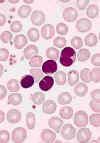 BLOOD LYMPHOCYTOSIS IN A PATIENT WITH PERTUSSIS. The lymphocytes in this blood smear from an 18-month-old
child with a Bordetella pertussis infection have lobulated nuclei. Lymphocytosis is characteristic of this disorder and the lymphocyte
morphology is often atypical. The cytology of the cells could be mistaken for neoplastic lymphocytes.
(Wright-Giemsa stain) © The Johns Hopkins Autopsy Resource
(JHAR). Image Archive.
BLOOD LYMPHOCYTOSIS IN A PATIENT WITH PERTUSSIS. The lymphocytes in this blood smear from an 18-month-old
child with a Bordetella pertussis infection have lobulated nuclei. Lymphocytosis is characteristic of this disorder and the lymphocyte
morphology is often atypical. The cytology of the cells could be mistaken for neoplastic lymphocytes.
(Wright-Giemsa stain) © The Johns Hopkins Autopsy Resource
(JHAR). Image Archive.
Tracheobronchial infections without pneumonia comprise a spectrum of disorders with different clinical implications: acute bronchitis, chronic bronchitis, and bronchiectasis. Acute bronchitis or “acute simple bronchitis” in otherwise-healthy persons is extremely common, usually of viral etiology, and a common reason for overuse of antibiotics. The term “acute infectious bronchitis” is sometimes used to distinguish this entity from other causes of cough, and the term “tracheobronchitis” is sometimes used for accuracy since the trachea is also inflamed. However, “chest cold” is probably the best term for daily practice since it implies that antibiotics are seldom necessary. Bordetella pertussis, the agent of whooping cough, is now recognized as a cause of acute bronchitis in adults. Infection by either Mycoplasma pneumoniae or Chlamydia pneumoniae accounts for many of the stubborn cases in which symptoms fail to resolve or recur soon after treatment has been discontinued.
Infection of the tracheobronchial mucosa causes local inflammation, increased secretion of mucus, and damage to ciliated cells. Symptoms result both from the inflammatory response and also from the interruption of the mucociliary blanket that normally cleanses the lower respiratory tract. Most cases of acute bronchitis (95% by some estimates) are caused by viruses. All of the common viruses affecting the upper respiratory tract have been implicated: rhinoviruses, coronavirus, respiratory syncytial virus, adenoviruses, coxsackieviruses, influenza viruses A and B, and parainfluenza virus. In two studies in which attempts were made to establish a precise diagnosis, the etiology was established in only 16% and 29% of cases with viruses being the most common causes.
Mycoplasma pneumoniae and Chlamydia pneumoniae probably
play minor roles in this illness, at least in most populations. However, M.
pneumoniae probably causes more cases of bronchitis than pneumonia, and
C. pneumoniae may be an important cause of acute bronchitis in college-aged
students. Whether S. pneumoniae,
H. influenzae, and M.
catarrahalis cause chest cold in otherwise-healthy persons is unclear, but
there is little support for the concept of "acute bacterial bronchitis" as a
community-acquired disease. Recently it has been emphasized that
Bordetella
pertussis can infect adults, even when vaccinated, providing a reservoir for
causing whooping cough among infants. Bordetella parapertussis causes a
protracted illness similar to whooping cough but without systemic toxicity.
Whether these observations can be generalized to other populations is
undetermined.
The onset is typically preceded by a
prodrome of
at least 24 hours with symptoms of
coryza and
pharyngitis. A dry cough, signifying early inflammation of the upper airway,
often evolves into a cough productive of moderate amounts of
mucopurulent
sputum. Fever, headache, myalgias, and
retrosternal
chest pain or discomfort may be present. Fever is most common when an influenza
virus or Mycoplasma pneumoniae is the causative agent. The patient rarely
looks toxic. Tracheal tenderness is often present.
Auscultation
may reveal a few coarse crackles with occasional wheezes in the chest, but there
are no signs of consolidation.
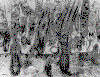 Transmission electron photomicrograph of a
hamster trachea ring infected with
M. pneumoniae. Note the orientation of the mycoplasmas through their
specialized tip-like organelle, which permits close association with the respiratory epithelium. M,
mycoplasma; m, microvillus; C, cilia.
Transmission electron photomicrograph of a
hamster trachea ring infected with
M. pneumoniae. Note the orientation of the mycoplasmas through their
specialized tip-like organelle, which permits close association with the respiratory epithelium. M,
mycoplasma; m, microvillus; C, cilia. Both images used with permission. From Baseman and Tully, Emerging Infection Diseases 3
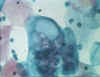 Figure Chlamydial inclusions © Bristol
Biomedical Archive. Used with permission
Figure Chlamydial inclusions © Bristol
Biomedical Archive. Used with permission
 Figure Chlamydial inclusions in an endothelial
cell © Bristol Biomedical Archive. Used with permission
Figure Chlamydial inclusions in an endothelial
cell © Bristol Biomedical Archive. Used with permission
Acute Infectious Exacerbations of Chronic Bronchitis
Chronic bronchitis is defined by the American Thoracic Society (ATS) as excessive sputum production with cough, present on most days for at least three months a year and not less than two successive years, without an underlying etiology such as tuberculosis or bronchiectasis. This common disorder, affecting up to 25% of the adult population, can lead to full-blown chronic obstructive pulmonary disease (COPD), the fourth-leading cause of death in the United States. The extent to which acute exacerbations are due to treatable infections remains controversial.
Chronic bronchitis is caused mainly by cigarette smoking. Air pollution, cold and damp climates, heredity, frequent lower respiratory tract infections, and immunodeficiency disorders (such as common variable hypogammaglobulinemia or isolated IgA deficiency) play a role in some patients. The essential feature is anatomic change in the larger airways, including an increased number of mucus-producing goblet cells and mucosal gland hypertrophy in the bronchial walls. Increased bronchial secretions and impaired ability to handle them lead to chronic cough and disabling complications.
Current opinion holds that most acute exacerbations of chronic bronchitis are caused by viruses or by non-infectious agents. Viruses have been found in as few as 7% to as many as 64% of cases in which they were sought. By conservative estimate viruses cause about one-third of cases, the more common ones being influenza viruses A and B, parainfluenza virus, coronaviruses, and rhinoviruses. Cultures of sputum often show non-typable strains of Haemophilus influenzae, Streptococcus pneumoniae, and/or Moraxella catarrhalis. However, the extent to which these bacteria explain exacerbations in a given patient is hard to determine since they often colonize the damaged lower respiratory tract on a more-or-less permanent basis. Evidence suggests that repeated episodes of bacterial infections—especially when caused by H. influenzae—contribute to deterioration of pulmonary function. S. aureus and aerobic gram-negative rods occasionally cause exacerbations of chronic bronchitis. The pathogens associated with "atypical pneumonia" such as M. pneumoniae, C. pneumoniae, and L. pneumophila probably cause fewer than 10% of exacerbations. Evidence to date suggests that Chlamydia pneumoniae is more strongly associated with the underlying chronic bronchitis than with its acute exacerbations. However, Chlamydia pneumoniae can cause a stubborn respiratory illness lasting several weeks or longer and tending to relapse after each course of antibiotics.
 Figure
FigureThis PA x-ray depicts atelectatic, and bronchiectatic changes in this child’s right upper pulmonary lung field.
Atelectasis (a collapse of the lung) can be due to bronchiectasis (an enlargement of the bronchial tubes) and a decrease in the effectiveness of their ciliated mucosal lining, which renders the lungs unable to clear themselves of clogging mucous build-up.
CDC/ Dr. Thomas Hooten
Bronchiectasis
Bronchiectasis is an acquired disorder characterized anatomically by abnormal dilatation of bronchi and bronchioles and clinically by chronic productive cough and frequent lower respiratory tract infections. Its prevalence fell dramatically after the introduction of broad-spectrum antibiotics and widespread immunization against measles and pertussis. Although bronchiectasis is now uncommon, it often goes undiagnosed until far-advanced. Newer imaging studies now enable earlier diagnosis, and our understanding of its causes continues to improve.
Cigarette smoking, the major cause of chronic bronchitis, plays little role in bronchiectasis except for predisposing to recurrent infections. The basic problem in bronchiectasis is permanent structural damage to the walls of bronchi and bronchioles brought about by the concerted action of (1) infection and (2) impairment of the pulmonary toilet, airway obstruction, and/or a defect in host defenses.
In the past, bronchiectasis was associated especially with frequent or severe lower respiratory infections during childhood. Bronchiectasis continues to be associated with such infections - especially necrotizing pneumonias in which treatment is delayed - but the list of known causes has expanded. Bronchiectasis can be the earliest clue to cystic fibrosis presenting during adolescence or early adulthood. Staphylococcus aureus, Pseudomonas aeruginosa, and Pseudomonas cepacia are often isolated from these patients. Mycobacterium avium-intracelluare complex (MAC) infection is not infrequently associated with bronchiectasis, especially in older women and/or thin women. Allergic bronchopulmonary aspergillosis often leads to bronchiectasis, which might be prevented by early recognition of this syndrome. Immunodeficiency disorders, both congenital (hypogammaglobulinemia) and acquired (AIDS) predispose to bronchiectasis. The dyskinetic cilia syndromes are sufficiently common (about 1 in every 20,000 to 60,000 persons) that a case is likely to occur in every medium-sized city.
Patients with advanced bronchiectasis experience daily cough productive of large amounts of mucopurulent, thick, tenacious sputum. However, most patients produce lesser amounts of sputum, at least during the early stages, and cough may be nonproductive ("dry bronchiectasis") or even absent. Dyspnea and hemoptysis are common. Patients often give a history of repeated respiratory infections and sometimes give a history of recurrent pleuritic chest pain. Hard crackles are heard locally over the lung fields in about 70% of patients. Rhonchi and widespread expiratory wheezes are also common. Clubbing is present in only about 3% of patients. Plain chest x-rays (PA and lateral views) are usually abnormal.
 Figure
FigureThis AP chest x-ray shows pneumonia of the left lower lobe with early consolidation, the etiology of which was unknown.
Pneumonia can be caused by a variety of agents including bacteria, viruses, and mycoplasmas, among others. Pneumonia remains an important cause of morbidity and mortality in the United States as both a primary, and secondary infection.
CDC/Dr. Thomas Hooten
 Figure
Figure
This anteroposterior x-ray reveals a bilaterally progressive
plague infection involving both lung fields.
The first signs of plague are fever, headache, weakness, and rapidly developing
pneumonia with shortness of breath, chest pain, cough, and sometimes bloody or
watery sputum, eventually progressing for 2 - 4 days into respiratory failure
and shock.
CDC/Dr. Jack Poland
Pneumonia accounts for an estimated 45,000 deaths in the United States each year. It is the sixth most common cause of death and the most common infectious cause of death. Since it is not a reportable disease, the precise incidence is unknown. Estimates suggest that 4 million cases occur each year, prompting 10 million physician visits and 600,000 to 1.2 million hospitalizations and adding $23 billion to health care costs. Data suggest a 28-fold increased cost for managing the disease on an inpatient basis (over $7,500 versus $264 for outpatient therapy). However, the mortality rate is 1% or less for patients managed as outpatients versus 14% to 25% for those admitted to the hospital. Physicians often overestimate the short-term mortality risk, but erring toward hospitalization is understandable given the potentially fatal nature of the disease.
Microorganisms can enter the lungs by aspiration, inhalation, or by way of the bloodstream (hematogenous pneumonia). Aspiration of bacteria that have colonized the oropharynx is by far the most common mechanism. Most humans aspirate small amounts of oropharyngeal secretions on a nightly basis. Microorganisms that are not removed by the mucociliary blanket are taken up and killed by pulmonary alveolar macrophages, the last line of defense. This process, called pulmonary clearance, is impaired by viral respiratory infections, tobacco smoke, chronic lung disease, alcohol, and many other factors associated with debilitating diseases. One or more chronic diseases are present in the majority of adult patients with pneumonia (58% to 89% of patients in various studies). Alcoholism predisposes to aspiration, but cigarette smoking is the main avoidable risk factor for community-acquired pneumonia in adults.
Inhalation of aerosolized particles is an important route of entry for many viruses including the influenza viruses and, most recently, the Hantaviruses. Bacteria that cause pneumonia by airborne transmission include M. tuberculosis, Yersinia pestis (plague), Bacillus anthracis (anthrax), and probably Legionella pneumophila (Legionnaire’s disease) and Francisella tularensis (tularemia). Spore-producing fungi such as Histoplasma capsulatum, Blastomyces dermatitidis, and Coccidioides immitis also cause inhalation pneumonia.
Hematogenous pneumonia classically develops from septic pulmonary emboli, frequently resulting in patchy or nodular bilateral pulmonary infiltrates sometimes accompanied by pleural effusions. In inner-city populations, a familiar scenario consists of bilateral pneumonia associated with S. aureus endocarditis on the tricuspid or pulmonic valves of injecting drug users. Another scenario consists of emboli from septic thrombophlebitis: for example, of the pelvic veins (pelvic inflammatory disease, septic abortion), internal jugular vein (the Lemierre syndrome) or any large vein where a catheter has been inserted. Hematogenous seeding of the lungs possibly explains some pneumonias caused by gram-negative bacteria and by unusually virulent organisms such as F. tularensis (tularemia).
The microbial cause of community-acquired pneumonia is usually difficult to determine. In prospective studies of patients requiring hospitalization, a cause is found in only 40% to 70% of cases. In primary care practice, a far greater fraction of cases are never diagnosed. Most of these cases respond to empiric therapy. Published data concerning the causes of pneumonia vary from one region to another, but some generalizations are possible. Mycoplasma pneumoniae has been determined to be the most common cause in some communities, when presumptive diagnoses were taken into account, followed by Streptococcus pneumoniae and Chlamydia pneumoniae. Adults with compromised host defenses are likely to have pneumococcal pneumonia, but can also have pneumonia due to H. influenzae, Moraxella catarrhalis, S. aureus, or aerobic gram-negative rods. There is wide agreement that S. pneumoniae is the most common cause of community-acquired pneumonia requiring hospitalization. An emerging and controversial area concerns the frequency of pneumonia caused by more than one microorganism. In one study, a second pathogen was found in about 10% of patients with pneumonia due to a conventional bacterial pathogen, but in 55% of patients in whom an "atypical" pathogen was found.
In 1938, the term "atypical pneumonia" was introduced to describe "an unusual form of tracheobronchopneumonia with severe constitutional symptoms". It later became customary to distinguish between "classical bacterial pneumonia" and "atypical pneumonia". This distinction was challenged during the 1990s when researchers found it difficult if not impossible to differentiate these illnesses on clinical grounds. Some authorities now suggest abandoning the term "atypical pneumonia". Others keep the term since it enriches our appreciation of the disease, forces us to consider unusual etiologies, and reminds us that the "atypical pneumonias" do not respond to β-lactam antibiotics. For these latter reasons and for the sake of clarity, we keep the terms here even while agreeing that in some cases it may be impossible to distinguish between "classic bacterial pneumonia" and "atypical pneumonia" in actual clinical practice.
"Typical pneumonia" is an alveolar disease whereas "atypical pneumonias" affects mainly the tracheobronchial mucosa and interstitium of the lung; hence, the different clinical manifestations.
Classical bacterial pneumonia begins with sudden onset of fever, chills, pleuritic chest pain, and productive cough. In the absence of impaired consciousness or inebriation, patients usually seek medical care within 6 hours of the onset of symptoms. Chills occur in about 50% and chest pain in about 30% of patients. Most patients are febrile although some, especially the elderly, may have normal or subnormal temperatures. The respiratory rate is usually increased. Physical examination often reveals signs of consolidation such as dullness to percussion, pectoriloquy, and egophony (e to a change). Lobar consolidation is present on chest x-ray in about one-third of patients. The white blood count is usually elevated with a shift-to-the-left. However, leukopenia rather than leukocytosis may be present and portends a poor prognosis.
Atypical pneumonia, on the other hand, usually begins gradually. The insidious onset is often brought out by asking, "When was the last time you were in your usual good health?" Constitutional symptoms are usually more prominent than the pulmonary symptoms. Chest pain is experienced as substernal discomfort. Cough is non-productive or productive of only scanty amounts of sputum. Relative bradycardia is frequently present. The trachea may be tender but the lung fields are essentially clear to auscultation, prompting one to be surprised by the extent of infiltrate present on chest x-ray. The white blood count is often normal or near normal. Modest elevation of liver enzymes (specifically, the aminotransferases - AST (SGOT) and ALT (SGPT) - is often present. Atypical pneumonia, in summary, seldom presents as an acute, life-threatening medical problem but forces the physician to expand the differential diagnosis.
Streptococcus pneumoniae remains the major cause of severe community-acquired pneumonia and, worldwide, a leading cause of death. It accounts for about two-thirds of cases of bacteremic pneumonia, and is the most common cause of pneumonia leading to hospitalization in all age groups. Some authorities believe that S. pneumoniae may cause up to one-half of all community-acquired pneumonias. There is concern that the incidence of pneumococcal disease may be increasing at the same time that drug resistance is becoming much more common. Primary care clinicians should strive to make pneumococcal vaccination an imperative for patients at increased risk.
S. pneumoniae is a common colonizer of the nasopharynx. Invasive pneumococcal disease occurs most often after a new serotype has been acquired, typically after an incubation of one to three days. Viral illness increases the incidence of disease presumably by interfering with normal host defenses. Risk factors for invasive pneumococcal disease include extremes of age, alcoholism, HIV disease, end-stage renal disease, sickle cell disease, diabetes mellitus, dementia, malnutrition, malignancies, diseases affecting B lymphocyte function (notably, multiple myeloma and hypogammaglobulinemia), and immunosuppressive disorders. Patients with asplenia are susceptible to fulminant pneumococcal disease. The pneumococcus does not invade cells as readily as do some of the other streptococci. However once in the lungs, it passes easily from one alveolus to another through the pores of Cohn until stopped by the dense connective tissue of a fissurehence the basis for lobar consolidation.
As classically described by previous generations of clinicians, S. pneumoniae causes a lobar pneumonia with by the sudden onset of fever with a single, hard-shaking chill, cough productive of rusty-colored mucopurulent sputum, and pleuritic chest pain. The patient presents with systemic toxicity including tachypnea. Physical examination reveals crepitant râles, tubular breath sounds, and signs of lobar consolidation (dullness to percussion, egophony with e to a change, and pectoriloquy). Today, however, pneumococcal disease is often a more subtle illness. Patchy infiltrates and bronchopneumonia are relatively common. It is often difficult to say precisely what represents pneumococcal pneumonia and what does not unless blood cultures are positive.
 Figure
FigureHaemophilus influenzae as seen using a Gram-stain technique.
During the flu outbreak of 1918 H. influenzae was termed Pfeiffer's Bacillus, where it was found in the sputum of many influenza patients, and thought to be the cause of influenza.
CDC
Among the numerous bacteria other than S. pneumoniae that sometimes cause acute community-acquired pneumonia, the most common are H. influenzae, S. aureus, Streptococcus pyogenes, miscellaneous aerobic gram-negative rods, and anaerobic "mouth flora" bacteria. Patients with these pneumonias often have significant underlying disease, severe pneumonia, or both. Therefore, hospitalization is usually indicated.
H. influenzae is a frequent cause of pneumonia in elderly patients and in patients with serious underlying diseases including chronic obstructive lung disease. The pneumonia usually has a patchy or segmental distribution characteristic of bronchopneumonia as opposed to lobar pneumonia. A sputum Gram’s stain showing small, pleomorphic gram-negative coccobacilli can be virtually diagnostic.
Staphylococcus aureus pneumonia, when community-acquired, tends to be an acute, fulminant about 1% of cases, except during influenza epidemics. Influenza virus infection markedly predisposes to staphylococcal colonization of the respiratory mucosa. Staphylococcal pneumonia tends to be a necrotizing process with abscess formation. The chest x-ray sometimes shows air pockets known as pneumatoceles, especially in children.
Streptococcus pyogenes (group A streptococcal) pneumonia is also uncommon except during influenza epidemics. This pneumonia is often accompanied by the rapid development of large empyemas. Chest tube drainage is often necessary, resulting in prolonged hospitalization.
Klebsiella pneumoniae is a relatively common cause of pneumonia in patients suffering from alcoholism. The pneumonia often assumes a lobar distribution. Classically, this pneumonia affects the upper lobes and causes a “bulging fissure” on chest x-ray. E. coli and other aerobic gram-negative rods are relatively common causes of pneumonia in the frail elderly. Pseudomonas aeruginosa, although a common cause of nosocomial pneumonia, is rarely associated with community-acquired pneumonia in patients without underlying lung disease or severe debility.
Pneumonia due to "mouth flora" bacteria - by which is meant a combination of anaerobic and aerobic bacteria with the anaerobes usually predominating - occurs most frequently in patients suffering from alcoholism and poor oral hygiene and results from aspiration. The sputum is usually copious and often foul smelling. "Mouth flora" pneumonia in an edentulous patient should prompt suspicion of underlying lung cancer. A foul odor to the breath is present in many but not all of these patients. This form of pneumonia is often associated with lung abscess and with empyema due to bronchopleural fistula.
Formerly known as the "Eaton agent", Mycoplasma pneumoniae is the most commonly identified cause of atypical pneumonia although its precise incidence is unknown. Various investigators have determined this microorganism to be the cause of 13% to 27% of community-acquired pneumonias. It can also cause hospital-acquired pneumonias, and it has caused as many as 50% of pneumonias during epidemics in closed populations. Mycoplasma pneumoniae pneumonia becomes less common after age 40, but older persons may experience more severe manifestations.
M. pneumoniae is a cell-wall-deficient organism with particular affinity for the respiratory tract epithelium. Many of the disease manifestations are now thought to be immune-mediated. Close, prolonged contact promotes transmission by respiratory secretions. There is currently interest in the extent to which M. pneumoniae accompanies other agents as a co-pathogen. In one study, an additional pathogen was found in about two-thirds of patients with M. pneumoniae pneumonia who required hospitalization; S. pneumoniae was most commonly found, but Legionella species and Chlamydia pneumoniae were also identified.
It is estimated that of persons infected with M. pneumoniae, about 20% are symptomatic, about 70% develop a mild respiratory illness (pharyngitis and/or tracheobronchitis), and fewer than 10% develop pneumonia. The disease occurs in all age groups including toddlers and the elderly but peaks between ages 5 to 15 years.
After an incubation period of about 3 weeks, symptoms begin gradually with fever, headache, malaise, chills, sore throat, substernal productive, and cough. The cough is initially non-productive, paroxysmal, and worse at night. It commonly becomes productive later in the illness. Physical examination is usually unimpressive. Bullous myringitis (inflammation of the tympanic membrane with bullae) is uncommon, occurring at most in about 5% of patients, but has a high positive predictive value for M. pneumoniae infection. More commonly there is mild tenderness over the paranasal sinuses, mild erythema of the posterior pharyngeal mucosa, soft cervical lymphadenopathy, and tracheal tenderness. Scattered râles and wheezes may be present but are usually unimpressive.
The white blood count is normal in 75% or more of cases. Thrombocytosis can occur as an acute-phase response. Liver enzymes, notably the aminotransferases (AST and ALT), are often mildly elevated. The chest x-ray commonly shows infiltrates that are much more extensive than one would have suspected from physical examination. The most common pattern is a peribronchial pneumonia in which thickened bronchial shadows are surrounded by streaky interstitial infiltrates and patchy atelectasis. Other patterns include nodular infiltrates and hilar lymphadenopathy. The lower lobes are most commonly involved, and pleural effusions - which can be especially severe in patients with sickle cell disease - occur in up to 20% of patients when carefully sought.
Extrapulmonary manifestations of M. pneumoniae pneumonia sometimes dominate the clinical picture and include hemolytic anemia, rashes including the life-threatening Stevens-Johnson syndrome, central nervous system complications (about 0.1% of patients, especially children), cardiac complications, and polyarthritis.
Chlamydia pneumoniae, described in 1986 as the TWAR agent, has been determined by some researchers to be the third or fourth most common cause of community-acquired pneumonia, explaining perhaps 10% to 14% of cases (up to 28% in some series). Pneumonia is recognized most frequently among the elderly, in whom it can be severe.
Chlamydia pneumoniae is classified as a bacterium on the basis of its cell wall and growth properties. Unlike most bacteria, however, it grows only as an intracellular parasite. Serologic studies suggest that most humans gain experience with C. pneumoniae at some point in their lives, although immunity is short-lived. About 50% of all persons have antibodies by age 20, and up to 75% of elderly persons are seropositive. It is also thought that most infections (up to 90%) are asymptomatic. Transmission is probably person-to-person by respiratory secretions.
After an incubation period of several weeks, most patients experience gradual onset of non-specific upper and lower respiratory symptoms including those of sinusitis, otitis, and pharyngitis. Sore throat with hoarseness is often prominent among the initial symptoms and tends to be the dominant symptom in college-aged persons. Symptoms of pneumonia tend to develop slowly. Often, patients have experienced symptoms for several weeks before seeking medical care. The history sometimes suggests a biphasic illness, as follows: (1) upper respiratory infection with sore throat that resolved, then (2) lower respiratory infection with cough.
The severity is age-dependent. Children under age five seldom have evidence of significant disease. University students often present with a 10-day history of sore throat or hoarseness with minimal fever. The mean age of patients with pneumonia is about 56 years. Ronchi and râles are present on physical examination more frequently than in M. pneumoniae pneumonia, even among patients who do not complain of cough. The white blood count is usually normal. Chest x-ray may show one or more infiltrates, the most common finding being a single, patchy, subsegmental infiltrate.
Wheezing is sometimes present. Accumulating evidence suggests that C. pneumoniae sometimes precipitates adult-onset asthma. Reported extrapulmonary manifestations of C. pneumoniae infection include meningoencephalitis, cerebellar dysfunction, Guillain-Barré syndrome, reactive arthritis, and myocarditis. The possibility that C. pneumoniae might cause coronary artery disease has received much attention. High antibodies to C. pneumoniae have been observed in patients with chronic obstructive lung disease, sarcoidosis, and lung cancer but an etiologic link is unclear.
About 100 to 200 cases of psittacosis are reported in the U.S. each year, but the true incidence is thought to be much higher. Mortality can be high if the diagnosis is not suspected.
Chlamydophila psittaci infects many and perhaps all species of birds, which may remain asymptomatic or show symptoms and signs of illness such as anorexia, dyspnea, and ruffled feathers. Strains of C. psittaci that are most virulent for humans tend to be those associated with psittacine birds (from the Latin psittacus, or parrot), such as parrots, parakeets, macaws, cockatoos, and budgerigars, and also with turkeys. Humans who develop psittacosis are commonly bird fanciers or work in poultry farms (notably, turkey farms), abattoirs, processing plans, pet shops, or veterinarians’ offices. The organism is usually acquired by inhalation, but human-to-human transmission occurs on rare occasions.
After an incubation period of 5 to 15 days, patients develop symptoms and signs of illness ranging in severity from mild to life threatening. Atypical pneumonia, the most characteristic form of the disease, is manifest by headache, fever, and non-productive cough. Chest x-ray is usually abnormal (75%) of cases, most commonly showing consolidation of one lower lobe. The radiographic findings are usually much more striking than the findings on auscultation of the chest. Psittacosis can also present as a typhoidal illness (fever, malaise, relative bradycardia, hepatosplenomegaly), a non-specific flu-like "viral syndrome", a mononucleosis-like syndrome, or as fever of unknown origin.
 Figure
FigureThis micrograph of a biopsied lung tissue specimen stained with the CDC's modified Dieterle silver impregnation procedure, revealed small, blunt, pleomorphic intracellular, and extracellular bacilli, which stain brown to black against a pale yellow background; Mag. 500x.
Dieterle’s stain is a preparation used when Legionella pneumophila bacteria are suspected. Uranyl nitrate is applied in order to first sensitize the slide mount, which is subsequently treated with gum mastic, followed by an incubation period, after which the slide is soaked in silver nitrate. Lastly, the slide is “developed” in a hydroquinone, sodium sulfite, acetone, formaldehyde, pyridine, and gum mastic bath. The Legionella pneumophila bacteria, if present, will stain black. CDC
 Figure
FigureThis silver-stained micrograph of a lung tissue specimen revealed the presence of Legionella pneumophila bacteria. The specimen was taken from a victim of the 1976 Legionnaires’ disease outbreak in Philadelphia.
CDC
 Figure
FigureLegionella pneumophila multiplying inside a cultured human lung fibroblast
CDC
 Figure
Figure
Anteroposterior x-ray reveals bilateral pulmonary infiltrates in
a patient with Legionnaires' disease CDC
First identified in 1976 during an outbreak at an American Legion Convention in Philadelphia, Legionnaire’s disease is now recognized as a relatively common cause of both community-acquired and hospital-acquired pneumonia. The incidence exhibits wide geographic variation - from less than 1% to more than 16% of community-acquired pneumonias - reflecting to a large extent the degree of contamination of water reservoirs by the causative organisms. Unlike pneumonias due to M. pneumoniae and C. pneumoniae, cases that can be treated on an outpatient basis tend to be the exception rather than the rule.
Legionella species are gram-negative bacteria that stain poorly and survive intracellularly. More than 40 species, and more than 60 serogroups, of Legionella are now recognized. Legionella pneumophila is the most commonly encountered species, causing at least 80% of clinical infections.
Legionellosis seems to be a disease of human progress brought about by devices that maintain water at warm temperatures and produce aerosols. In water, the organisms multiply within amebas; in humans they multiply within alveolar macrophages. The disease is spread by water rather than by person-to-person contact. Contamination of water sources has been associated with numerous outbreaks in settings ranging from inner-city hospitals to luxury cruise liners.
Clinically severe cases of Legionnaire’s disease tend to occur in persons with compromised host defenses, most often in the setting of chronic obstructive pulmonary disease, immunosuppression, or advanced age. A mild form of Legionellosis, known as Pontiac fever, is a self-limited disease presenting as fever, malaise, headaches, and chills without pneumonia. Pontiac fever resolves within a few days without antibiotic therapy. Legionnaire’s disease, the more familiar and more severe form of Legionellosis, affects persons of all ages and presents with symptoms that overlap those of "classic bacterial" and "atypical" pneumonia.
After an incubation period of 2 to 10 days, patients experience the onset of fever, headache, anorexia, malaise, and myalgia. At this point, respiratory symptoms are usually not prominent, the cough being only minimally productive. Some patients have chest pain and, if the sputum is blood-tinged, pulmonary embolism is often suspected. Gastrointestinal symptoms with nausea, vomiting, diarrhea, and abdominal pain can also dominate the clinical picture. Alternatively, neurologic symptoms can be the presenting complaint, variably manifested as headache, lethargy, and change in mental status.
Fever is usually present. Relative bradycardia is found more often in older patients and in those with severe pneumonia. Examination of the chest usually shows râles and, later in the illness, signs of consolidation. The peripheral blood commonly shows leukocytosis and thrombocytopenia. Hyponatremia (serum sodium < 130 mEq/L) is more common in Legionnaire’s disease than in most other pneumonias. Hypophosphatemia also occurs. There is frequently evidence of liver and renal dysfunction. Hematuria and proteinuria are common. There is no characteristic feature on chest x-ray. The most common pattern is a patchy infiltrate involving one lobe, which progresses to consolidation. Infiltrates can assume a diffuse or interstitial pattern, and pleural effusions are common.
![]() Return to the Infectious Disease Section of Microbiology and Immunology On-line
Return to the Infectious Disease Section of Microbiology and Immunology On-line
This page last changed on
Tuesday, February 17, 2015
Page maintained by
Richard Hunt