|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
|
VIROLOGY - CHAPTER TEN
PICORNAVIRUSES - PART ONE
ENTEROVIRUSES AND GENERAL FEATURES OF PICORNAVIRUSES
Dr Richard Hunt
Professor
Department of Pathology, Microbiology and Immunology
University of South Carolina School of Medicine
Columbia, South Carolina
|
|
En
Español |
|
|
Let us know what you think
FEEDBACK |
|
|
|
SEARCH |

 |
|
|
Appendix
Acute Flaccid Myelitis (AFM): Update on Disease Symptoms and Potential Etiologic
Agent(s) |
|
CASE REPORTS
Poliovirus Infections in Four
Unvaccinated Children --- Minnesota, August--October 2005
|
Picornaviruses are the small positive strand RNA viruses
that do not have a lipid
membrane. They have a naked nucleocapsid that is about 30mm in diameter. Pico
means small, hence small RNA viruses or picornaviruses. Based on
a number of properties including sequence homologies and acid
sensitivity, there are nine genera within the Picornaviridae.
Five of these infect humans:
- Enteroviruses
- Rhinoviruses
- Hepatoviruses
- Parechoviruses
- Kobuviruses
Parechoviruses were formerly classified among the Echoviruses and
cause gastrointestinal and respiratory tract infections, and
occasionally cases of encephalitis and flaccid paralysis. Kobuviruses
also cause gastoenteritis.
|
| |
|
Table
1 Genera of Picornaviruses |
| Genera that infect
humans |
Enterovirus
Polio
Coxsackie A and B
Echo
Other enteroviruses
|
Diseases of
the human (and other) alimentary tract (e.g. polio virus) |
| Rhinovirus |
Disease of the
nasopharyngeal region (e.g. common cold virus) |
| Hepatovirus |
Human
hepatitis virus A |
| Parechovirus |
Formerly echoviruses 22 and 23. Disease
of alimentary and respiratory tract |
| Kobuvirus |
Aichi virus is the type species |
| Genera that infect other
animals |
| Cardiovirus |
Mainly found
in rodents
Murine
encephalomyocarditis, Theiler's murine encephalomyelitis virus |
| Aphthovirus |
Foot and mouth
disease in cloven footed animals |
| Erbovirus |
The Erbovirus genus has a
single species, Equine rhinitis B virus. It is divided into two
serotypes |
| Teschovirus |
From Teschen disease in pigs - virulent
porcine polioencephalomyelitis which has high morbidity and mortality |
| Others |
Drosophila C
virus, cricket paralysis virus |
|
| |
|
Table
2 Enteroviruses |
|
Virus family |
Serotypes |
| Polio |
1 - 3 |
| Coxsackie A |
1 - 22, 24 |
| Coxsackie B |
1 - 6 |
| Echovirus |
1 - 9, 11 - 27,
29 - 34 |
| Hepatitis A |
Enterovirus 72 |
| Other
Enteroviruses |
68 - 71 |
|
| |
|
Table
3 Properties of Rhino- and Entero-viruses |
|
|
pH
sensitivity |
Optimum
growth temperature |
Detergent
sensitivity |
Serotypes |
Transmission |
Site of
primary infection |
|
Rhino
viruses |
labile to acid
pH |
33 degrees C
(approx) |
|
>100 |
aerosol |
upper
respiratory tract |
|
Entero
viruses |
resistant to
acid pH |
37 degrees C
(approx) |
Resistant |
72 |
oro-fecal |
gut |
|
 Poliovirus Poliovirus
© Jim Hogle, From Grant, R.A.,
Cranic, S. and Hogle, J.M. (1992) Radial Depth Provides the Cue. Curr.
Biol. 2: 86-87. From
Virus World, Sgro, J-Y
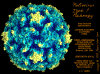 Poliovirus © J-Y Sgro, Used with permission.
From
Virus World
Poliovirus © J-Y Sgro, Used with permission.
From
Virus World
 Transmission electron micrograph of poliovirus type 1.
CDC/Dr. Joseph J. Esposito
jje1@cdc.gov
Transmission electron micrograph of poliovirus type 1.
CDC/Dr. Joseph J. Esposito
jje1@cdc.gov
 Negatively stained preparation of a typical Enterovirus, Coxsackie B, and seen by
transmission electron microscopy. Wadsworth Center, New York
State Department of Health
Negatively stained preparation of a typical Enterovirus, Coxsackie B, and seen by
transmission electron microscopy. Wadsworth Center, New York
State Department of Health
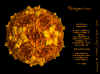 Cardiovirus: Molecular surface of
Mengovirus, radially depth cued, as solved by X-ray crystallography
© J-Y Sgro. From:
VirusWorld. Used with permission
Cardiovirus: Molecular surface of
Mengovirus, radially depth cued, as solved by X-ray crystallography
© J-Y Sgro. From:
VirusWorld. Used with permission
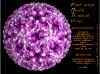 Aphthovirus: Molecular surface of Foot and Mouth Disease Virus, radially depth cued, as solved by X-ray crystallography
© J-Y Sgro. From:
VirusWorld. Used with permission
Aphthovirus: Molecular surface of Foot and Mouth Disease Virus, radially depth cued, as solved by X-ray crystallography
© J-Y Sgro. From:
VirusWorld. Used with permission
Figure 1
- Micrographs of picornaviruses
|
GENERAL FEATURES OF PICORNAVIRUSES
Picornaviruses have an icosahedral nucleocapsid (figure 1). There are 60 identical
subunits (vertices) which contain five protomers. Each protomer is made up of
one copy of four proteins, named VP1, VP2, VP3 and VP4. These proteins are made
as a single polypeptide (polyprotein) which is cleaved by cellular proteases.
The order of formation of the individual viral proteins is important in the
assembly of the virus. The single strand of positive-sense RNA (messenger RNA
sense) can act as a messenger RNA once it enters the cytoplasm and uncoating has
occurred. The polio
virus RNA comprises 7741 bases with a large 5' leader sequence of 743 bases that
does not code for viral protein (untranslated region). The open reading frame then extends to near the
3' end. After the open reading frame of 7000 bases, there is a short sequence
before the poly A tract. The poly A tract of polio RNA is encoded in the genome,
unlike the situation with cellular mRNAs where it is
added post-transcriptionally. There is another way in which picornavirus RNA differs from a
typical mRNA. The latter have a methylated cap structure at the 5' end, whereas
picornaviruses have a viral protein called VPG. The large 5' leader sequence has
considerable secondary structure that comes about by intramolecular base pairing
and one of these structures is the internal ribosome entry site (IRES) which allows
this RNA to bind to cytoplasmic ribosomes. In the normal cellular process,
initiation of protein synthesis is different and follows what are known as the Kozak rules. The initiation AUG codon in the polio virus open
reading frame is preceded by eight other AUGs.
Receptor binding
Different picornaviruses have different receptors, among which are some
intercellular cell surface adhesion molecules (ICAMs). The expression of these
molecules determine tissue tropism. Coxsackievirus (a type of enterovirus, see
later) and most rhinoviruses bind to ICAM-1, an adhesion glycoprotein expressed
on the surfaces of a variety of cells (epithelial, endothelial, fibroblasts).
Polio virus binds to another cell surface glycoprotein known as CD155 (the
poliovirus receptor). When the
virus binds to its receptor, the VP4 protein is released from the protomer. This
allows the escape of the viral RNA from the nucleocapsid when the virus in
internalized into the endocytic pathway. In the endosome, the
nucleocapsid disassembles in the acid environment. Protein synthesis is
detectable with 15 minutes of infection.
Translation and protein processing
The picornavirus RNA binds to ribosomes and makes a single polypeptide,
therefore the virus has just one gene. This polyprotein has regions that have
proteolytic activity (they are cysteine proteases) that cleave the polyprotein
to three precursor proteins (P1, P2, P3). P1 is cleaved to a VP0, VP1 and Vp3
plus a leader peptide of unknown function. VP0 gives rise to VP2 and VP4. P2 and
P3 do not give rise to viral structural proteins. One of the proteins that comes
from P3 is the VPG that is found at the 5' end of the viral RNA while other
proteins from this precursor are the viral replicase and enzymes that modify the
behavior of the host cell. P2 is also cleaved to give other cell-modifying
proteins. Details of some of the cleavages are still vague.
Once the various viral proteins have been made in the infected cell, the
replicase (also call a transcriptase or protein 3Dpol) copies the viral plus
sense RNA to negative sense RNA. Other viral proteins are also involved in this
process. As new positive strand RNAs are made, they can also be translated into
more viral protein. There may be as many as half a million copies of viral RNA
per cell. Some of the proteolytic events outlined above take place as the
nucleocapsid is assembled. This is especially the case with the VP0 cleavage to
VP2 and VP4. P1 protein is the precursor that gives rise to the four structural
proteins of the nucleocapsid. Five copies of P1 first associate. Endoproteolysis
then occurs to form VP0, VP1 and VP3. Twelve of these pentamers than associate
to form an empty capsid (procapsid). The viral RNA now associates with the
capsid and at the same time, VP0 is cleaved. Release is by lysis of the host
cell.
At the same time as viral protein synthesis is occurring, host cell protein
synthesis is shut off. The host cell mRNAs however remain fully functional when
assayed in an experimental system, so selective degradation of cell mRNAs is not
the reason for protein synthesis inhibition. One way host cell protein synthesis
occurs is via the
cleavage of initiation factor eIF-4, one of the cap binding proteins of the host
cell's ribosomes so that cellular
mRNAs cannot bind to the ribosomes. Association with cap-binding proteins is a
prerequisite for the translation of most cellular RNAs. Thus, only uncapped
messages such as that of the picornavirus are translated. Note that most viruses
express capped RNAs similar to normal mRNA and so this mechanism of shutting
down host protein synthesis is not available to them. The viral proteins also
change the permeability of the host cell, altering the ionic composition of the
cell and inhibiting cell mRNA association with ribosomes. Moreover, the large
number of copies of viral RNA simply out-compete the cell's mRNAs.
|
| |
ENTEROVIRUSES
Pathology
|
Table
4 Human diseases caused by enteroviruses |
|
|
Poliovirus |
Coxsackie A
virus |
Coxsackie B
virus |
Echovirus |
Enterovirus
(other) |
|
Asymptomatic
infection |
yes |
yes |
yes |
yes |
yes |
|
Meningitis |
yes |
yes |
yes |
yes |
yes |
|
Paralysis |
yes |
yes |
yes |
yes |
?* |
|
Febrile
exanthems |
no |
yes |
yes |
yes |
yes |
|
Acute
respiratory disease |
no |
yes |
yes |
yes |
yes |
|
Myocarditis |
no |
yes |
yes |
yes |
no |
|
Orchitis |
no |
no |
yes |
yes |
no |
| *
Enterovirus-D68 (EV-D68) can replicate in blood and may damage the
central nervous system. It has been detected in cerebrospinal fluid of
patients with acute flaccid paralysis.
There have been reports of children
hospitalized with muscle weakness or paralysis, usually in their arms
and legs. They were tested for poliovirus, West Nile virus, and
enteroviruses. About half of the children had EV-D68 in their nose
secretions; usually, EV-D68 affects the respiratory system and it is not
yet known if this respiratory infection is linked to their muscle
weakness.
|
|
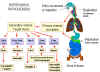 Pathogenesis of enteroviruses. Cox = Coxsackie virus A or B, Hep A =
hepatitis A virus, Echo = echovirus, Polio = poliovirus
Pathogenesis of enteroviruses. Cox = Coxsackie virus A or B, Hep A =
hepatitis A virus, Echo = echovirus, Polio = poliovirus
Figure 2
- Enterovirus pathogenesis |
Enteroviruses are spread via the fecal-oral route. The ingested viruses
infect cells of the oro-pharyngeal mucosa and lymphoid tissue (tonsils) where
they are replicated and shed into the alimentary tract.. From here they may pass
further down the gastrointestinal tract. Because of the acid stability of these
viruses, they can pass into the intestine and set up further infections in the
intestinal mucosa. The virus also infects the lymphoid tissue (Peyer's
patches) underlying the intestinal mucosa. At these sites, the virus replicates
and are shed into the feces, often for months after the primary infection. In the
primary viremic phase, the virus also enters the bloodstream at low levels. The
tissues that are then infected depend on the expression of the correct
receptors. For example, CD155, the polio virus receptor, is expressed in spinal
cord anterior horn cells, dorsal root ganglia, skeletal muscle, motor neurons
and some cells of the lymphoid system. Expression of CD155 within embryonic
structures giving rise to spinal cord anterior horn motor neurons may explain
the restrictive host cell tropism of polio virus for this cellular compartment
of the central nervous system. There are three polio virus serotypes and all of
them bind to the CD155 receptor protein. For unknown reasons, polio virus does
not spread to the cells of the central nervous system in all patients. The
Coxsackie virus receptor (which also binds adenovirus) is a surface protein with
two immunoglobulin-like domains is more widely expressed.
At this stage symptoms may occur and the patient may experience fever and
malaise. A secondary viremia may occur at this time. The spread of the virus
form the gastro-intestinal tract and the secondary viremia that occurs about 10
days after the initial infection leads to a humoral and cell-mediated immune
response (the latter being of less importance). This rapidly limits the further
replication of the virus in all tissues except the GI tract because the virus
must pass through extracellular space to infect another cell. In the GI tract
replication may be sustained for several weeks even though a high titer of
neutralizing antibody is achieved. The cells in which this replication occurs
are not known and it is unclear why replication occurs in the presence of the
neutralizing antibody. Although each group of enteroviruses share a receptor,
the various serotypes of a group are usually not blocked by group-specific
antibodies even though it would be expected that they would have a common
receptor binding site. The v reason for this appears to be that the cell
receptor protein binds to a viral protein at the bottom of a canyon into which
the cell protein can fit but an antibody cannot.
|
| |
DISEASES CAUSED BY ENTEROVIRUSES
Most patients infected with an enterovirus remain asymptomatic but in small
children benign fevers caused by unidentified enteroviruses are relatively
common (non-specific febrile illness). Many outbreaks of febrile illness
accompanied by rashes are also caused by enteroviruses.
|
|
|
POLIOVIRUS
Poliomyelitis means inflammation of the
gray (poliós) spinal cord (myelós).
It is also known as infantile paralysis.
Our first record of poliomyelitis comes from an Egyptian stele from the 18th
dynasty (1580-1350 BCE) showing a victim of the disease with a withered leg
(figure 3).
Poliovirus caused about 21, 000 cases of paralytic poliomyelitis in the
United States each year in the 1940's - 50's prior to the introduction of the
Salk (inactivated) and Sabin (attenuated) vaccines. The height of the epidemic occurred in 1950 when there
were 34,000 cases. By 2000, the number of cases of paralytic polio in the US was
fewer than 10 and these were the result of the attenuated (Sabin) vaccine
reverting to virulence (see
Vaccines). Today, the attenuated vaccine is no
longer used and the number of vaccine-associated polio cases in the US is close
to zero. However, the ease with which the attenuated virus reverts to virulence,
as a result of genetic drift (mutation), means that if people who were
vaccinated with the attenuated live virus continue to shed it in feces,
the problem of vaccine-associated disease will remain. Most people clear the
attenuated strain of virus but a few people with immunological problems do not;
for example, people with
hypogammaglobulinaemia (a B-cell deficiency
disorder) do not mount a humoral antibody response to poliovirus. They become
asymptomatic chronic long-term excreters of the vaccine-derived virus (in one
case for more than 20 years) and their virus can infect people who have not been
vaccinated or who have lost immunity.
|
 Egyptian stele from the 18th dynasty showing a victim of polio with a
withered leg
Egyptian stele from the 18th dynasty showing a victim of polio with a
withered leg
Figure 3 |
There are three serotypes of polio virus. Most disease results from type 1
polio virus. Since the disease is spread by fecal contamination, infections are
more common where unsanitary conditions prevail but many children in these areas
have asymptomatic infections that lead to life-long immunity. In contrast, in
western countries naturally acquired immunity as a result of asymptomatic
exposure is reduced and subsequent exposure to the virus may lead to severe
disease in later life. Ironically, therefore, the disease of polio (as opposed
to infection) is a disease of development and better sanitation.
Asymptomatic polio infection
Infection by polio virus is, in most cases (more
than 90%),
asymptomatic. This occurs when
the replication of the virus is restricted to the gastro-intestinal tract (as is the case with
the attenuated vaccine strain). Exactly why many polio infections are
asymptomatic is controversial but probable variables include the size of the innoculum of the virus, the size of the resulting viremia, the virulence of the
infecting virus, and the presence of circulating antibodies. It is clear from
clusters of cases that the same virus can cause very different outcomes in
different patients from no symptoms to mild fever with diarrhea to flaccid paralysis.
|
 Iron lung ward in the 1950's
Iron lung ward in the 1950's
 Paralyzed child in an iron lung
Paralyzed child in an iron lung
 Child with polio sequelae
© WHO
Child with polio sequelae
© WHO
 Victims of paralytic polio © WHO
Victims of paralytic polio © WHO
Figure 4
- Paralytic polio |
Abortive poliomyelitis (minor illness)
The first symptomatic result of polio infection is febrile disease and occurs
in the first week of infection. The patient may exhibit a general malaise which
may be accompanied by vomiting, a headache and sore throat. This is abortive
poliomyelitis and occurs in about 5% of infected individuals
Non-paralytic poliomyelitis
Three or four days later a stiff neck and vomiting, as a result of muscle
spasms, may occur in about 2% of patients. This is similar to aseptic
meningitis. The virus has now progressed to the brain and infected the meninges.
Paralytic polio
About 4 days after the end of the first minor symptoms, the virus has spread
from the blood to the anterior horn cells of the spinal cord and to the motor
cortex of the brain. The degree of paralysis depends on the which neurons are
affected and the amount of damage that they sustain. The disease is more
pronounced in very young and very old patients. In spinal paralysis one or more
limbs may be affected or complete flaccid paralysis may occur (figure 4). In bulbar
paralysis cranial nerves and the respiratory center in the medulla are affected
leading to paralysis of neck and respiratory muscles. There is no sensory loss
associated with the paralysis. The degree of paralysis may increase over a
period of a few days and may remain for life or there may be complete recovery
over period of 6 months to a few years. In bulbar poliomyelitis, death may also
ensue in about three quarters of patients, especially when the respiratory
center is involved. Patients were able to survive for a while using an iron lung
to aid respiration (figure 4). The morality rate of paralytic polio is 2-3%
Post-polio syndrome
This afflicts victims of an earlier polio virus infection but the virus is no
longer present. It may occur many years after the infection and involves
loss of function in affected muscles, perhaps as a result of further neuron loss.
|
|
|
| |
COXSACKIE VIRUSES
There are many infections caused by Coxsackie viruses, most of which are
never diagnosed precisely. Coxsackie type A usually is associated with surface
rashes (exanthems) while type B typically causes internal symptoms (pleurodynia,
myocarditis) but both can also cause paralytic disease or mild respiratory tract
infection. The latter can be caused by several Coxsackie virus types and by
Echoviruses and the symptoms are much like a rhinovirus infection.
Meningitis
Enteroviruses are the major cause of viral meningitis. Both Coxsackie virus A
and B can cause aseptic meningitis which is so-called because it is not of
bacterial origin. Viral meningitis typically involves a headache, stiff neck,
fever and general malaise. Lymphocyte pleocytosis of the cerebrospinal fluid is
often observed. Most patients recover from the disease unless encephalitis
occurs although there may be mild neurological problems. The disease is most
prevalent in the summer and fall.
Herpangina
Coxsackie virus A can cause a fever with painful ulcers on the palate and
tongue leading to problems swallowing and vomiting. Treatment of the symptoms is
all that is required as the disease subsides in a few days. Despite its name,
the disease has nothing to do with herpes or the chest pain known as angina.
|
|
|
Hand, foot and mouth disease
This is an exanthem (that is, a rash) caused by Coxsackie type A16. Symptoms include
fever and blisters on the hands, palate and feet. Again, it subsides in a few
days. Many other exanthems may be caused by Coxsackie virus or Echoviruses.
|

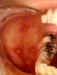 Hand, Foot and Mouth Disease © Bristol Biomedical
Image Archive. Used with permission
Hand, Foot and Mouth Disease © Bristol Biomedical
Image Archive. Used with permission
Figure 5 |
Myocarditis
Coxsackie virus A and B (and also Echoviruses) can cause myocarditis in
neonates and young children. Fever, chest pains, arrhythmia and even cardiac
failure can result. Mortality rates are high. In young adults, an acute benign
pericarditis may also be cause by Coxsackie viruses.
Bornholm disease (Pleurodynia, the Devil's
Grippe)
Usually caused by Coxsackie A, these upper respiratory tract infections can
result in fever and sudden sharp pains in the intercostal muscles on one side of
the chest. There may also be pain in the abdomen and vomiting. The incubation
period is 2 to 4 days and symptoms subside after a few days although relapses
can occur.
Other enterovirus diseases
Non-specific febrile disease can be caused by several enteroviruses These
infections are among the most common reasons that small children are admitted to
hospital in order to rule out a bacterial cause. The virus can spread
transplacentally or from maternal fecal material and is most severe in infants
born to mothers who contract the viral infection shortly before giving birth or
in infants who contract the virus after birth. This is because the mother has
not had time to developing a protective immune response and pass protective
antibody to the infant. Admissions peak in the late summer/fall. Disease
normally resolves but can be of consequence in the very young. Coxsackie B virus
may result in severe neonatal disease including hepatitis, meningitis,
myocarditis and adreno-cortical problems. Infections often spread through
nurseries and are difficult to stop because of the resistance of the virus to
disinfecting agents.
|
| |
Acute hemorrhagic conjunctivitis is caused by Coxsackie A24 and enterovirus
70. The disease resolves in a week or two.
Hepatitis
Hepatitis A virus, the major cause of viral hepatitis, is also an enterovirus
but it will be dealt with in the hepatitis section.
PREVENTION OF PICORNAVIRUS DISEASE
One of the more important feats of 20th
century medicine was the development of highly effective vaccines that have
almost eradicated poliomyelitis from the world. Vaccination is therefore the
major means of control of this virus and the major vaccines are discussed in the
section Vaccines.
There are no vaccines for Coxsackie virus or other
enteroviruses. In most
cases, enterovirus infections are not life-threatening and management of
symptoms are all that is required. However, certain patients particularly those
with deficient humoral immunity, acquire serious infections. These include
chronic enterovirus meningoencephalitis, neonatal enterovirus sepsis,
myocarditis, vaccine- associated or wild-type polio virus infection, post-polio
muscular atrophy syndrome, enterovirus encephalitis and bone marrow transplanted
patients with an enterovirus infection. Treatment with antibody preparations
(immune globulin) has resulted in stabilization of the conditions of some of
these patients but the virus persists and few of these patients survived their
infections. Recently, however, treatment with pleconaril (related to the WIN
drugs) has shown a response that is temporally related to therapy (for further
information on pleconaril and the WIN drugs, see
anti-viral
chemotherapy).
DIAGNOSIS
It is frequently difficult to diagnose an enterovirus disease from symptoms
alone and epidemiology is used. For example, if
there is a local outbreak of viral meningitis in the summer or fall, the patient is
likely to be infected with Coxsackie A or B.
|
|
|
 Return to the Virology section of Microbiology and Immunology On-line
Return to the Virology section of Microbiology and Immunology On-line
 Return to the Home Page of Microbiology and Immunology On-line
Return to the Home Page of Microbiology and Immunology On-line
This page last changed on
Friday, December 28, 2018
Page maintained by
Richard Hunt
|

 Poliovirus
Poliovirus Pathogenesis of enteroviruses. Cox = Coxsackie virus A or B, Hep A =
hepatitis A virus, Echo = echovirus, Polio = poliovirus
Pathogenesis of enteroviruses. Cox = Coxsackie virus A or B, Hep A =
hepatitis A virus, Echo = echovirus, Polio = poliovirus
 Egyptian stele from the 18th dynasty showing a victim of polio with a
withered leg
Egyptian stele from the 18th dynasty showing a victim of polio with a
withered leg
 Iron lung ward in the 1950's
Iron lung ward in the 1950's

 Hand, Foot and Mouth Disease © Bristol Biomedical
Image Archive. Used with permission
Hand, Foot and Mouth Disease © Bristol Biomedical
Image Archive. Used with permission







