|
x |
x |
|
 |
 |
|
INFECTIOUS
DISEASE |
BACTERIOLOGY |
IMMUNOLOGY |
MYCOLOGY |
PARASITOLOGY |
VIROLOGY |
|
|
VIROLOGY -
CHAPTER EIGHT
VACCINES: PAST SUCCESSES
AND FUTURE PROSPECTS
FROM SMALLPOX TO COVID-19
Dr Richard Hunt
Professor
University of South Carolina School of Medicine
Columbia
South Carolina
|
|
En
Español |
|
SHQIP - ALBANIAN |
|
TURKISH |
Let us know what you think
FEEDBACK |
|
SEARCH |

 |
|
|
|
See also:
APPENDIX
Reduction in the incidence of certain diseases after the introduction of
vaccination

Polio Virus (From the Hogle Lab at Harvard, URL unknown)
Many of the images in the smallpox part of this file come from
Fenner, Henderson, Arita et
al. Smallpox and its Eradication. 1988 Geneva, World Health Organization and were
assembled by Laura Gregorio in her essay The Smallpox Legacy, Pharos. Fall 1996
|
INTRODUCTION
What is a vaccine?
Vaccines are harmless agents, perceived as enemies.
They are molecules, usually but not necessarily proteins, that elicit an immune
response, thereby providing protective immunity against a potential pathogen.
While the pathogen can be a bacterium or even a eukaryotic protozoan, most
successful vaccines have been raised against viruses and here we shall deal
with anti-viral vaccines. Vaccines may consist of a purified protein, nucleic
acid or a
complex of molecules or even a whole bacterium or virus.
Immunity to a virus normally depends
on the development of an immune response to antigens on the surface of
a virally infected cell or on the surface of the virus particle itself. Immune responses to internal antigens
often play little role in immunity.
Thus, in influenza pandemics, a novel surface glycoprotein acquired as a result of
antigenic shift characterizes the new virus strain against which the population
has little or no immunity. This new strain of influenza virus may, nevertheless, contain internal
proteins that have been in previous influenza strains.
Surface glycoproteins are often referred to as protective antigens. To make a
successful vaccine against a virus, the nature of these surface antigens
must be known
unless the empirical approach of yesteryear is to be followed. It should be
noted, however, that a virally-infected cell displays fragments of internal
virus antigens on its surface and these can elicit a cytotoxic T cell response
that acts against the infected cell.
There may be more than one surface glycoprotein on a virus and one
of these may
be more important in the protective immune response than the others; this antigen must be
identified for a logical vaccine that blocks infectivity. For example, influenza virus has
a neuraminidase and a hemagglutinin on the surface of the virus particle.
It is the hemagglutinin that provokes neutralizing immunity because it is the
protein that attaches the virus to a cell surface receptor and the neutralizing
antibody interferes with virus binding to the cell.
In addition to blocking cell to virus
attachment, other factors can be important in the neutralization of viruses; for
example, complement can lyze enveloped
virions after opsonization by anti-viral antibodies.
In this chapter, we deal mostly with anti-viral vaccines, although
there are also successful anti-bacterial vaccines (see
here).
|
|
WEB RESOURCES
Common Misconceptions about Vaccination and how to respond to them |
Major sites of viral infection
In order to develop a successful vaccine, certain
characteristics of the viral infection must be known. One of these is the site
at which the virus enters the body. Three major sites may be defined:
Virus families in this group are:
picornaviruses;
measles
virus; mumps virus;
herpes simplex virus;
varicella virus;
hepatitis
A and B viruses
Virus families in this group are
hepatitis B
virus; alphaviruses; flaviviruses; bunyaviruses
IgA-mediated local immunity is very important in
the first two categories. There is
little point in having a good neutralizing humoral antibody in the circulation when the
virus replicates, for example, in the upper respiratory tract. Clearly, here secreted
antibodies are important.
Thus, we need to know:
-
The viral antigen(s) that elicit neutralizing antibody
-
The cell surface antigen(s) that elicit neutralizing antibody
-
The site of replication of the virus
|
| |
Types of vaccines
There are five basic types of vaccine in use today
-
Killed vaccines: These are preparations of the normal (wild
type) infectious, pathogenic virus that has been rendered non-pathogenic,
usually by chemical treatment such as with formalin that cross-links viral
proteins.
-
Attenuated vaccines: These are live virus particles that grow in
the vaccine recipient but do not cause disease because the vaccine virus has
been altered (mutated) to a non-pathogenic form; for example, its tropism has
been altered so that it no longer grows at a site that can cause disease.
-
Sub-unit vaccines: These are purified components of the virus,
such as a surface antigen.
-
DNA vaccines: These are usually harmless viruses into which a
gene for a protective antigen has been spliced. The protective
antigen is then made in the vaccine recipient to elicit an immune response
-
mRNA vaccines: These are the RNA-coding sequence of a
protective (usually surface) antigen that is translated by the cells of
the vaccinee after injection and expressed on the surface of transfected
cells.
Problems in vaccine development
There are many problems inherent in developing a good protective
anti-viral vaccine. Among these are:
-
Different types of virus may cause similar
diseases -- e.g. the common cold. As a result, a single vaccine will not be possible
against such a disease
-
Antigenic drift and shift -- This is especially true of
RNA viruses
and
those with segmented genomes
-
Large animal reservoirs. If these occur, re-infection after elimination from
the human population may occur
-
Integration of viral DNA. Vaccines will not work on latent virions
unless they express antigens on the cell surface. In addition, if the vaccine virus integrates
into host cell chromosomes, it
may cause problems (This is, for example, a problem with the possible use of
anti-HIV vaccines based on
attenuated virus strains)
-
Transmission from cell to cell via syncytia - This is a problem for potential
AIDS vaccines since the virus may spread from cell to cell without the virus
entering the circulation.
-
Recombination and mutation of the vaccine
virus in an attenuated vaccine.
Despite these problems, anti-viral vaccines have, in some cases, been
spectacularly successful (see
addendum) leading in one case (smallpox) to
the elimination of the disease from the human population. The smallpox
vaccine is an example of an attenuated vaccine, although not of the
original pathogenic smallpox virus. Another successful vaccine is the
polio vaccine which may lead to the elimination of this disease from the
human population soon. This vaccine comes in two
forms. The Salk vaccine is a killed virus, while that developed by
Albert Sabin is a live attenuated virus vaccine. Polio is presently restricted to south Asia
(Pakistan and Afghanistan). Although smallpox is the only human
disease that has been eradicated using vaccination, it is likely that one animal
disease has also been eradicated. Rinderpest (cattle plague or steppe murrain)
is a viral disease with high mortality that infects cattle and other ruminants
and causes fever, diarrhea and lymphoid necrosis. It is a member of the measles
family (Family: Paramyxoviridae; Genus: Morbillivirus) and was eradicated using
a live attenuated vaccine. In 2010, the Food and Agriculture Organization
reported that no case of rinderpest had been diagnosed for nine years. It is
thus the only disease of agricultural livestock that has been successfully
eradicated.
|
 Figure 1b
Figure 1b
Mary Wortley Montague
The work of art depicted in this image
and the reproduction thereof are in the
public domain
worldwide. The reproduction is part of a
collection of reproductions compiled by The Yorck
Project. The compilation copyright is
held by
Zenodot Verlagsgesellschaft mbH
and licensed under the
GNU Free Documentation License. |
PAST SUCCESSES
Smallpox
(Variola)
Smallpox is a devastating and disfiguring
disease that is highly infectious. It is caused by variola virus (also
known as smallpox virus), a member of the orthopoxvirdae (figure
2A). The disease of smallpox has been known for thousands of years and
probably originated in Asia. It spread westwards into the middle east
and among its victims was Pharaoh Rameses V (figure 2B). The disease may
have reached Europe with the crusaders. Smallpox was introduced to the
New World by European colonists and caused devastating epidemics in the
indigenous population who had no natural immunity. Indeed, some early
colonists used smallpox as a biological weapon again the original
inhabitants of North and South America.
Smallpox is characterized by numerous
pustules containing infectious virus all over the body (figure 2 C and
D). The fatality rate is more than one quarter of infected patients
infected by the most serious form caused by Variola major.
Another form of smallpox caused by Variola minor has a much lower
fatality rate (up to 5%).
The first attempts to control smallpox
occurred in the 10th
century and used variolation
(so called because small pox virus is Variola). In variolation (figure
2E), material (scabs) was obtained from the pustules
of an infected person who did not die of the disease. This person, therefore,
had a milder form of smallpox as a result of a naturally occurring variant. This
material was used to infect another person who usually also got a milder
disease. If the person did not die, there was lifelong immunity. Another
reason for the success of variolation was that virus in the scabs was
less virulent because it had been partially desiccated and was complexed
with and inactivated by antibodies from the donor.
The fatality
rate of variolation was about 1 - 2% and so it was still a dangerous procedure.
This technique was used in
Pakistan, Ethiopia and Afghanistan until 1970. Variolation
was widespread in England in 1700s where it was introduced by the wife of
the British Ambassador to Turkey, Mary Wortley Montague (figure 1b).
|
|
Figure
2

A. Smallpox virus
Copyright
1994 Veterinary Sciences
Division
Queen's University Belfast
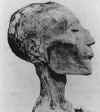 B. The mummified head of
Ramses V (died 1157 BCE)
with rash that
is probably the result
of smallpox
B. The mummified head of
Ramses V (died 1157 BCE)
with rash that
is probably the result
of smallpox
|
 C.
Infant with smallpox
C.
Infant with smallpox
 D. Smallpox lesions on skin of trunk. Photo taken in Bangladesh.
CDC/James Hicks
D. Smallpox lesions on skin of trunk. Photo taken in Bangladesh.
CDC/James Hicks
 E. Powdered smallpox scabs were inhaled to
protect against smallpox in Chinese medicine
E. Powdered smallpox scabs were inhaled to
protect against smallpox in Chinese medicine
|
| |
|
| Figure 3

A. Edward Jenner
 B. Dr Jenner about to
vaccinate a child
B. Dr Jenner about to
vaccinate a child |
 C. Blossom the
cow C. Blossom the
cow
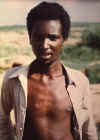 D. The last known person in the world to have a natural case of smallpox. Variola minor in 23-year-old Ali Maow
Maalin, Merka, Somalia
D. The last known person in the world to have a natural case of smallpox. Variola minor in 23-year-old Ali Maow
Maalin, Merka, Somalia
CDC
|
| |
In
1796, Edward Jenner
(figure 3A), who was at the time experimenting with variolation, discovered
vaccination using vaccinia virus, the
agent of cowpox (vacca is the Latin for cow).
Jenner was a physician living in rural Gloucestershire in the west of
England and it was widely known at that time that people who
contracted cowpox (such as dairy maids) appeared to gain protective
immunity against the much more virulent smallpox. Jenner vaccinated a Mr Phipps
(who worked for him) and own son
(figure 3B) with cowpox from a cow called Blossom (figure 3C), and then challenged them with virulent smallpox.
Both vaccinees were, fortunately, protected. Jenner's original virus is
not the vaccinia that was used in smallpox vaccinations until recently. The
vaccine virus may have
arisen as recombinant from cowpox or horse pox. For a long time the
vaccine virus was maintained in horses or buffalo.
The last case of natural smallpox
in the U.K. occurred in the 1930s; the last in the U.S.A. was in the 1940s.
The last natural case in the world was in Somalia and occurred in October
1977 (figure 3D). Although the virus had been eliminated in the wild,
smallpox was retained in laboratories and as a result of a laboratory accident there was a fatal case in
Birmingham, England in 1978 when a medical photographer died. This person
was the last person to die of smallpox in the world.
Worldwide stocks were reduced
to laboratories in the United States and the Soviet Union. It is not known
whether infectious virus from the Russian laboratory was distributed after the
dissolution of the Soviet Union.
The eradication of smallpox has been one of the great triumphs
of public health. There are several reasons for this:
-
There is no animal reservoir for
variola, only humans are infected by this virus
-
Once a person has been infected by the
virus, there is lifelong immunity, although this may not be the case with people
immunized using the vaccine strain
-
Subclinical cases are rare and so an
infected person can be identified and isolated
-
Infectivity does not precede overt symptoms,
that is there is no
prodromal phase
-
There is only one Variola serotype and
so the vaccine is effective against all virus strains
-
The vaccine is very effective
-
There has been a major commitment by
the World Health Organization and governments to smallpox erradication.
|
 Figure 4. Figure 4.
Louis Pasteur
 Figure 5.
Figure 5.
Rabies virus |
RabiesAlmost a century after Jenner's pioneering
work on smallpox, in 1885 Louis Pasteur (figure 4) and Emile Roux developed the first
vaccine against rabies (figure 5) (rabhas, Sanskrit: to do violence).
Pasteur discovered that if he took spinal cord material from a dead rabid rabbit
and kept it for a period of 15 days in a dry atmosphere (a flask containing
potassium hydroxide) and then injected it into a dog, the latter did not get
rabies. He developed a protocol in which he carried out the same procedure with
spinal cord tissue that had been kept in a dry atmosphere for less and less time
(each was separated by an interval of two days), until he finally injected
spinal tissue containing virulent virus (only a day or two in the flask). He
found that the dog was then immune to rabies.
Pasteur successfully treated a boy (Joseph Meister) bitten by a rabid dog
sixty hours earlier with this protocol in which he used successively more
virulent virus. In fact, according to Pasteur, the rabies in the final
inoculation was more virulent than that of ordinary canine rabies. Fortunately,
Mr Meister survived both the initial bites and the virulent virus!
Current anti-rabies vaccines are not prepared in the way that Pasteur used.
Human Diploid Cell Vaccine (HDCV) is made in tissue culture using normal human
WI-38 fibroblasts. The rabies virus is purified by passage through a filter and
inactivated by beta-propriolactone. This inactivated virus vaccine is used
almost exclusively in the developed world for pre- and post-vaccination of
rabies. Purified Chick Embryo Vaccine (PCEC) is also purified virulent virus. It
is made by ultracentrifugation and also inactivated by beta-propriolactone.
These vaccines
give a high titer of neutralizing antibody after 10 days. When used properly,
they can confer 100% protection.
There is also a live attenuated vaccine (Flury strain) that is grown in chick
embryos and is for use in animals only.
A recombinant anti-rabies vaccine (VRG, Raboral) has been made by inserting
the gene for the surface glycoprotein of rabies into vaccinia virus, the virus
used in smallpox vaccinations. The recombined virus appears safe for humans but
is used for treating wild animals since (because it is a live virus) it can give
herd immunity. The vaccine virus is stable to elevated temperatures and can
be delivered orally. It is therefore fed to animals in food baits. Raboral V-RG®
is approved for immunization of raccoons and coyotes, two of the most
significant wildlife carriers of rabies in North America.
|
| |
|
|
|
Poliomyelitis
In
western countries, wild type
polio is no longer a problem but it is still
endemic in Pakistan
and
Afghanistan (figures 6). However, wild poliovirus has been
imported into some countries that have stopped transmission of
indigenous virus and outbreaks can result from these
importations. A number of countries continue to be affected by such
outbreaks. Most of these are in the “wild poliovirus importation
belt” – a number of countries stretching from west Africa to central
Africa and the Horn of Africa (figure 6).
Until the 1950s, when anti-polio vaccination became routine,
summer outbreaks of polio were common in western countries, often spread
via the oral-fecal route while using swimming pools. These outbreaks led
to widespread paralytic polio that necessitated help in breathing and the
use of "iron lungs" (figure 7).
Anti-polio vaccines
There are two types of polio
vaccine, both of which were developed in the 1950s. The first, developed
by Jonas Salk, is a formalin-killed preparation of normal wild type polio
virus. This is grown in monkey kidney cells and the vaccine is given by
injection. It elicits good humoral (IgG) immunity and prevents transport
of the virus to the neurons where it would otherwise cause paralytic
polio. This vaccine is the only one used in some Scandinavian countries
where it completely wiped out the disease.
A second vaccine was developed
by Albert Sabin. This is a live attenuated vaccine that was produced
empirically by serial passage of the virus in cell culture. This resulted
in the selection of a mutated virus that grew well in culture and, indeed,
in the human gut where the wild type virus grows. It cannot, however,
migrate to the neurones. It replicates a normal infection since the virus
actually grows in the vaccinee and it elicits both humoral and
cell-mediated immunity. It is given orally, a route that is taken by the
virus in a normal infection since the virus is passed from human to human
by the oral-fecal route. This became the preferred vaccine in the
United States, United Kingdom and many other countries because of it ease
of administration (often on a sugar lump), the fact that the vaccine virus
replicates in the gut, and only one administration is needed to get good
immunity (though repeated administration was usually used). In addition,
the immunity that results from the Sabin vaccine lasts much longer that
that by the Salk vaccine, making fewer boosters necessary. Since it
elicits mucosal immunity (IgA) in the gut (figure 10), the Sabin
vaccine has the potential to wipe out wild type virus whereas the Salk
vaccine only stops the wild type virus getting to the neurons.
The attenuated Sabin vaccine, however,
came with a problem: back mutation. This may result from recombination between
wild type virus and the vaccine strain. Virulent virus is frequently isolated
from recipients of the Sabin vaccine. The residual cases in countries
that used the attenuated live virus vaccine
(about 8 per year in the US until recently; figure 9a, b and c) resulted from mutation of the vaccine strain to virulence. About half of these cases
were in vaccinees and half in contacts of vaccinees. Paralytic polio arises in 1 in 100 cases of
infection by wild type virus and 1 in 2.4 million initial vaccinations as a result of back reversion
of the vaccine to virulence. This was deemed acceptable as the use of the attenuated virus means that
the vaccine strain of the virus still replicates in the body and gives gut immunity via IgA.
The vaccinee who has received killed
Salk vaccine still allows wild type virus to replicate in his/her gastro-intestinal tract, since the
major immune response to the injected killed vaccine is circulatory IgG (figure
10). As
noted above, this
vaccine is protective against paralytic polio since, although the wild type
virus can still replicate in the vaccinee's gut, it cannot move to the nervous
system where the symptoms of polio are manifested. Thus, wild type virus is
unlikely to die out in populations who have received only the killed vaccine since
it would be shed in the feces. It should be noted, however, that studies in The
Netherlands during a polio outbreak in 1992 (among people who had refused the vaccine)
showed that immunity produced by the Salk vaccine did prevent circulation of wild type
virus in the general population.
An additional problem of using a live attenuated vaccine is that
preparations may contain other pathogens from the cells on which the virus was
grown. This was certainly a problem initially because the monkey cells used to
produce the polio vaccine were infected with
simian virus 40 (SV40) and this was also in the
vaccine. SV40 is a polyoma virus and has the potential to cause cancer. It appears,
however, not to have caused problems in vaccinees who inadvertently received it.
There have been some allegations that the original attenuated polio vaccine used
in Africa may have been contaminated with
human immunodeficiency virus
(HIV). This has been found not to be the case. Of course, there can also be similar
problems with the killed vaccine if it is improperly inactivated. This has also
occurred.
Current recommendations concerning polio vaccines
Once the only polio cases in the US were vaccine-associated,
the previous policy of solely using the Sabin vaccine was reevaluated.
At first, both vaccines were recommended with the killed vaccine first and then the attenuated vaccine. The
killed vaccine would stop the revertants of the live vaccine giving trouble by moving to
the nervous system. Thus, in 1997 the following protocol was recommended:
To reduce the vaccine associated cases (8 to 10 per year), the CDC
Advisory Committee on Immunization Practices (ACIP) has recommended (January 1997) a regimen of two
doses of the injectable killed (inactivated: Salk) vaccine followed by two doses of the
oral attenuated vaccine on a schedule of 2 months of age (inactivated), 4 months
(inactivated), 12-18 months (oral) and 4-6 years (oral). Currently four doses of the oral
vaccine are typically administered in the first two years of life. It is thought that the
new schedule will eliminate most of the cases of vaccine-associated disease. This regimen
has already been adopted by several European countries and some of Canada .
The regimen of polio vaccination was
subsequently amended again in 2000:
To eliminate the risk for
Vaccine-Associated Paralytic Poliomyelitis, the ACIP recommended an all-inactivated poliovirus vaccine
(IPV) schedule for routine childhood polio vaccination in the United States. As of January 1, 2000, all children should receive four doses of IPV at ages 2 months, 4 months,
6-18 months, and 4-6 years.
In 2009, the recommendations were further
revised:
Three different combination vaccines
containing IPV have been licensed for routine use in the
United States. Because of potential confusion in using
different vaccine products for routine and catch-up
immunization, ACIP recommends the following:
The 4-dose IPV series should continue to be administered at
ages 2 months, 4 months, 6--18 months, and 4--6 years.
The final dose in the IPV series should be administered at
age ≥4 years regardless of the number of previous doses.
The minimum interval from dose 3 to dose 4 is extended from
4 weeks to 6 months.
The minimum interval from dose 1 to dose 2, and from dose 2
to dose 3, remains 4 weeks.
The minimum age for dose 1 remains age 6 weeks.
|
|
CASE REPORT
Poliovirus Infections in Four
Unvaccinated Children --- Minnesota, August--October 2005 |
Figure
6 - Polio Statistics
Comparison of worldwide incidence in 1988, 1998, 2004 and 2013
 Polio - 1988 WHO
Polio - 1988 WHO
 Polio - 1998 WHO
Polio - 1998 WHO
 Polio - 2004
Polio - 2004
WHO
 Polio - 2013
Polio - 2013
polioeradication.org
 Polio 2020
Polio 2020
Our World in data |
|
Figure
7 (right) -
Poliomyelitis
|
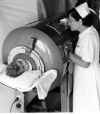 A. Child in iron lung
A. Child in iron lung
WHO
 B. Iron lung ward
B. Iron lung ward
 C. Child with polio sequelae
C. Child with polio sequelae
WHO
 D. Child with polio sequelae
D. Child with polio sequelae
WHO
 E. Victims of polio
E. Victims of polio
WHO
|
| |
|
 Figure 8
Figure 8
Total reported cases in
Sweden and Finland (1950-76) which use the killed vaccine only
developed by Jonas Salk. The Salk vaccine is injected |
 Figure 9a
Figure 9a
Reported (cases per 100,000 population) cases of paralytic
poliomyelitis in the United States, 1951-1992, which initially used the killed
Salk vaccine. This was subsequently replaced by the live attenuated oral vaccine
developed by Albert Sabin. The Sabin vaccine is swallowed. It is often
given on a sugar lump
 Figure 9b
Figure 9b
Poliomyelitis in the US 1980-1995
CDC
 Figure 9c
Figure 9c
Vaccine-associated paralytic polio - VAPP in US 1964-1995
CDC
 Figure 10
Figure 10
Secretory antibody (nasal and gut IgA) and serum antibody (serum IgG, IgM
and IgA) in response to killed polio vaccine (left) administered by
intramuscular injection and to live attenuated polio vaccine (right)
administered orally
|
| |
 Figure 10a
Figure 10a
Global rotavirus deaths, 2013
WHO
 Figure 10b
Figure 10b
Countries with the highest numbers of rotavirus deaths in children
WHO
 Figure 10c
Figure 10c
Rotavirus mortality in children under five in 2013
WHO
|
Rotavirus disease: An initial
problem but later success
Rotaviruses
are found worldwide and almost very child in the world is infected by
rotaviruses by the age of five. These viruses cause major gastroenteritis and
diarrhea-associated hospitalization and over 215,000 deaths in 2013 in children under five years of age.
In 2013, 37% of deaths in children resulted from diarrhea (figure 10a).
The virus is spread by the oro-fecal route.
According to WHO, five
countries (India, Nigeria, Pakistan, the Democratic Republic of the Congo
and Angola) accounted for more than half of all rotavirus
disease deaths under age five (figure 10b and c). Symptoms include: fever,
vomiting, diarrhea and abdominal pain.
Seroprevalence studies show that antibody is present in most infants
by age 3 years.
Prior to the introduction in the United States of widespread vaccination
in 2006, there were up to three million cases of rotavirus infection per
year. In about 1 to 2.5% of cases, there was severe dehydration. This
resulted in 20 to 60 deaths of children under five each year. In
addition, there were 50,000 to 70,000 hospitalizations and over 500,000
visits to doctors’ offices per year.
Since the introduction of vaccination there has been a drop in
rotavirus-related hospitalizations by up to 86 percent. It is likely
that vaccination has also protected non-vaccinated infants by limiting
circulating infection. Deaths have also been markedly reduced. In 2008,
there were an estimated 14 deaths from rotavirus disease in the United
States and fewer than 10 in the United Kingdom compared to 98,621 in
India.
Rotashield and intussusception
Reassortant vaccines are created by genetic reassortment in
which non-human rotavirus strains express the antigens of human
rotaviruses on their surface. The non-human strains replicate but do
not cause disease and are of low pathogenicity in humans. A live,
tetravalent rhesus-human reassortant vaccine (Rotashield - Wyeth
Laboratories) was first licensed for use in infants in August 1998.
It contained human G types 1, 2, 4, and simian G type 3. However,
post-licensure surveillance indicated a possible relationship
between the occurrence of intussusception 3 to 20 days after the
vaccine was administered, especially the first dose (15 cases/1.5
million doses were reported). Intussusception is a rare
intestinal obstruction that occurs when the intestine folds on
itself or telescopes into itself resulting in reduced blood supply.
It is most common among young children. The most common place in the
intestine for intussusception to occur is where the small intestine
joins the colon. However, it can occur in many parts of the
intestine. With prompt treatment, almost all patients fully recover.
It is more common in boys than in girls.
Use of the Rotashield vaccine was suspended and it was eventually
removed from the market in October 1999, when studies confirmed the
link between vaccination and intussusception.
RotaTeq
RotaTeq (Merck) is a live oral vaccine licensed in the United
States in 2006. It contains five reassortants (WC3 bovine rotavirus
strain with surface proteins of the G1-4 and P1A human serotypes). It
does not contain preservatives or thimerosal. Three doses are given
at 2, 4 and 6 months of age with the minimum age for the first dose
of 6 weeks. The series should not be initiated after 12 weeks. The
efficacy of the RotaTeq vaccine is high with 98% reduction in severe
rotavirus gastroenteritis within the first year of vaccination and a
96% reduction in hospitalization rate. There is also a 74 and 71%
reduction of rotavirus gastroenteritis within the first and second
years after vaccination.
Rotarix
Rotarix (Glaxo Smith Klein) is a human, live attenuated rotavirus
vaccine which contains a rotavirus strain of G1P[8] specificity. It
is used for the prevention of rotavirus gastroenteritis caused by G1
and non-G1 types (G3, G4, and G9) when administered as a 2-dose
series in infants and children.
Both of these rotavirus vaccines are very effective (85% to 98%) at
preventing infection-associated gastroenteritis and diarrhea. CDC
recommends routine vaccination of infants with either of the two
available vaccines. Both are administered orally.
- RotaTeq® (RV5). This is given in 3 doses at ages 2 months, 4
months, and 6 months
- Rotarix® (RV1). This is given in 2 doses at ages 2 months
and 4 months.
Other vaccines include Rotavac (India), Rotavin (Vietnam) and
Langzhou Lamb (China).
|
| |
OTHER ANTI-VIRAL VACCINES
There are a number of other commonly used anti-viral vaccines and
these are listed below
|
TABLE 1
Common currently used anti-viral vaccines |
|
Virus |
Vaccine Type |
Micrograph
|
CDC Links |
|
Polio (Salk) |
Inactivated |
 Transmission electron micrograph of poliovirus type 1.
CDC/Dr. Joseph J. Esposito jje1@cdc.gov
Transmission electron micrograph of poliovirus type 1.
CDC/Dr. Joseph J. Esposito jje1@cdc.gov |
Updated Recommendations of the Advisory Committee on Immunization Practices (ACIP)
Regarding Routine Poliovirus Vaccination (2009) |
|
Polio (Sabin) |
Attenuated |
|
Rabies |
Current human vaccine is inactivated.
There is an attenuated vaccine for animal use. |
Rabies Virus
New York State Department of Health |
ACIP Recommendations
Use of a Reduced (4-Dose) Vaccine Schedule for Postexposure Prophylaxis to
Prevent Human Rabies (2008) |
|
Mumps |
Attenuated |

Mumps Virus
CDC PHIL |
Use of Combination Measles, Mumps, Rubella, and Varicella Vaccine
Recommendations of the Advisory Committee on Immunization Practices (ACIP) |
|
Measles |
Attenuated |

Measles Virus
CDC PHIL |
|
Rubella |
Attenuated |
Rubella virus
CDC PHIL |
|
Influenza |
Inactivated |
Influenza virus
Copyright 1994 Veterinary Sciences Division
Queen's University Belfast |
CDC
Vaccine Information
Prevention and Control of Influenza with Vaccines: Recommendations of the
Advisory Committee on Immunization Practices (ACIP), 2011
|
|
Hepatitis A |
Inactivated |
|
Prevention
of Hepatitis A Through Active or Passive Immunization.
Recommendations of the Advisory Committee on Immunization Practices (ACIP),
2006 |
|
Hepatitis B |
Subunit |
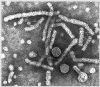
Hepatitis B Virus
Copyright Dr Linda M Stannard, University of Cape Town, South Africa, 1995. |
Hepatitis B
Vaccine Recommendations (2005, 2006, and 2011)
Part 1 - Infants, Children, & Adolescents
Part 2 - Adults |
|
Varicella |
Attenuated |

Varicella Virus
John Curtin School of Medical Research
Australian National University
Canberra,
Australia.
Micrograph:
Dr Frank Fenner |
Prevention of Varicella
Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007 |
|
Rotavirus |
Attenuated |

Rotavirus
Copyright 1994 Veterinary Sciences Division , Queen's
University, Belfast |
Prevention of Rotavirus Gastroenteritis Among Infants and
Children Recommendations of the Advisory Committee on Immunization Practices
(ACIP), 2009
Rotavirus and Intussusception |
|
Yellow Fever |
Attenuated |

Yellow fever virus
CDC PHIL |
Yellow Fever Vaccine
Recommendations of the Advisory Committee on Immunization Practices (ACIP),
2010 |
|
Human Papilloma |
Subunit |
|
Quadrivalent Human Papillomavirus Vaccine
Recommendations of the Advisory Committee on Immunization Practices (ACIP),
2007 |
|
Japanese Encephalitis |
Inactivated |
|
Japanese Encephalitis Vaccines
Recommendations of the Advisory Committee on Immunization Practices (ACIP),
2010 |
Varicella
|
Attenuated |

Varicella (Chickenpox) Virus
CDC PHIL |
Prevention of Varicella.
Recommendations of the Advisory Committee on Immunization Practices (ACIP),
2007 |
|
Herpes Zoster |
Attenuated |
|
Prevention of Herpes Zoster.
Recommendations of the Advisory Committee on Immunization Practices (ACIP),
2008 |
For more information on
anti-HIV (AIDS) vaccines go
here
|
|
|
|
| |
KILLED VERSUS ATTENUATED VACCINES
Attenuated Vaccines
Attenuation is usually achieved by passage of the virus in foreign
host such as embryonated eggs or tissue culture cells. From among the many
mutant viruses that exist in a population (especially so in RNA viruses), some
will be selected that have a better ability to grow in the foreign host (higher virulence). These tend
to be less virulent for the original host. To produce the Sabin polio vaccine, attenuation was only
achieved with high inocula and rapid passage in primary monkey kidney cells. The
virus population became overgrown with a less virulent strain (for humans) that could grow well in
non-nervous (kidney) tissue but not in the central nervous system. Non-virulent strains of all three polio types
have been produced
for the vaccine.
Molecular basis of attenuation
We do not know
the basis of attenuation in
most cases since attenuation was achieved empirically. The empirical foreign-cell passage method causes many mutations in a virus and
it is difficult to determine which are the important mutations. Many attenuated viruses
are temperature-sensitive (that is, they grow better at 32 - 35 degrees than 37 degrees) or cold adapted
(they may grow at temperatures as low as 25 degrees). In the type 1 polio virus attenuated vaccine strain,
there are 57
nucleotide changes in the genome, resulting in 21 amino acid changes . One third
of the mutations are in the VP1
gene (this gene is only 12% of genome). This suggests that attenuation results from changes in
surface proteins of the virus
An attenuated nasal
vaccine for influenza
(FluMist®) has been developed (see below). This contains
cold-adapted vaccine strains of the influenza virus that have been grown
in tissue culture at progressively lower temperatures. After a dozen or
more of these passages, the virus grows well only at around 25° and in vivo growth is restricted to the upper respiratory tract. Studies showed that
influenza illness occurred in only 7 percent of volunteers who received
the intra-nasal influenza vaccine, versus 13 percent injected with
trivalent inactivated influenza vaccine and 45 percent of volunteers who
were given placebo. Both vaccine comparisons with placebo were
statistically significant.
|
| |
Advantages of attenuated vaccines
-
They activate all phases of immune system.
They elicit humoral IgG and
local IgA (figure 8)
-
They raise an immune response to all protective antigens.
Inactivation, such as by formaldehyde in the case of the Salk vaccine, may alter antigenicity
-
They offer more durable immunity
and are more cross-reactive. Thus, they stimulate antibodies against multiple
epitopes which are similar to those elicited by the wild type virus
-
They cost less to produce
-
They give quick immunity in majority of vaccinees
-
In many cases (e.g. polio and adenovirus vaccines), administration
is easy
-
These vaccines are easily transported in
the field
-
They can lead to elimination
of wild type virus from the community
Disadvantages of Attenuated vaccine
-
Mutation. This may lead to reversion to virulence
(this is a major disadvantage)
-
Spread to contacts of the vaccinee who have not consented to be
vaccinated (This could also be an advantage in communities where vaccination is not 100%)
-
Spread of the vaccine virus
that is not standardized and may be mutated
-
Sometimes there is poor "take" in tropics
-
Live viruses are a problem in
immunodeficiency disease patients
|
| |
Advantages of inactivated vaccine
-
They give sufficient humoral immunity if boosters given
-
There is no mutation or reversion (This
is a big advantage)
-
They can be used with immuno-deficient patients
-
Sometimes they perform better
in tropical areas
Disadvantages of inactivated vaccines
-
Some vaccinees do not raise immunity
-
Boosters tend to be needed
-
There us little mucosal / local immunity
(IgA). This is important (figure 8)
-
Higher cost
-
In the case of polio, there is
a shortage of monkeys
-
In the case of smallpox, there
have been failures in inactivation leading to immunization with virulent virus.
|
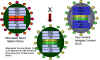 Figure 11 Attenuated influenza vaccine strain using a cold-sensitive mutant that
can be reassorted with new virulent strains
Figure 11 Attenuated influenza vaccine strain using a cold-sensitive mutant that
can be reassorted with new virulent strains |
New methods of vaccine production
Selection for mis-sense
Conditional lethal mutants. Temperature-sensitive mutants in influenza A and RSV
have been made by mutation with 5-fluorouracil and then selected for temperature
sensitivity. In the case of influenza, the temperature-sensitive gene can be reassorted in the laboratory to yield a virus
strain with the coat of the strains circulating in the population and the inner
proteins of the attenuated strain. Cold
adapted mutants can also be produced in this way. It has been possible to obtain
mis-sense mutations in all six genes for non-surface proteins.
The
attenuated influenza vaccine, called FluMist, uses a cold-sensitive mutant that can be reassorted with any new virulent influenza strain that appears
(figure 11). The reassorted
virus will have the genes for the internal proteins from the attenuated virus
(and hence will be attenuated) but will display the surface proteins of the new
virulent antigenic variant. Because this is based on a live, attenuated virus,
the customization of the vaccine to each year's new flu variants is much more
rapid than the process of predicting what influenza strains will be
important for the coming flu season and combining these in a killed vaccine.
|
| |
Synthetic peptides
Injected peptides which are much smaller than the original virus protein raise
an IgG response but there is a problem with poor antigenicity. This is
because the epitope may
depend on the conformation of the protein in the virus as a whole. A non-viral example that has achieved some
limited success is a prototype anti-malarial vaccine.
|


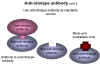 Figure 12 Anti-idiotype antibodies
Figure 12 Anti-idiotype antibodies |
Anti-idiotype
vaccines
An antigen binding site in an antibody is a reflection of the three-dimensional structure of
part of the antigen, that is of a particular epitope. This unique amino acid structure
in the antibody is known as the idiotype which can be thought of as a
mirror of the epitope in the antigen.
Antibodies (anti-ids) can be raised against the idiotype by injecting the antibody into another animal. This gives us an anti-idiotype
antibody and this, therefore, mimics part of the three dimensional structure of the antigen,
that is, the epitope (figure 12).
This can be used as a vaccine. When the anti-idiotype antibody is injected into
a vaccinee, antibodies (anti-anti-idiotype antibodies) are formed that
recognize a structure similar to part of the virus and might potentially neutralize the virus. This happens: Anti-ids raised against
antibodies to hepatitis B S antigen elicit anti-viral antibodies.
|
| |
Recombinant DNA
techniques
Attenuation of virus
Deletion mutations can be made that are large enough
that they are unlikely to revert (though suppression of the mutation remains a
problem. This has been seen in some of the Nef deletion mutants developed as
potential anti-HIV vaccines). Another problem with this approach in some
vaccines is that the virus could still retain other unwanted characteristics such as oncogenicity
(e.g. with adenovirus, herpes virus, HIV).
Single gene approach
(usually a surface glycoprotein of the virus)
A single gene (for
a protective
antigen) can be expressed in a foreign host. Expression vectors are used to make large amounts of
antigen to be used as a vaccine. The gene could be expressed in and the protein
purified from bacteria using a fermentation process, although lack of post-translational processing
by the bacteria is a problem. Yeast are better for making large amounts of
antigen for vaccines since they process glycoproteins in their Golgi bodies in a manner more
similar to mammals. An example of a vaccine in which a viral protein is
expressed in and purified from yeast is Gardasil, an anti-human papilloma
virus vaccine that is very effective in preventing cervical cancer. The
current hepatitis B vaccine is also this type. A similar anti-human
papilloma vaccine, Cervarix, is made by expressing viral genes recombined
into a bacculovirus and expressed in insect cells.
Expression of the SARS-CoV-2 S protein in bacculovirus
is also used in the preparation of the Novavax COVID-19 vaccine (see
below) which has been shown to be highly effective, possibly because of
the use of an effective proprietary adjuvant.
These vaccines have many
of the disadvantages of a killed vaccine. This approach has been used to make several
potential HIV vaccines but they provoke little cell-mediated immunity.
Cloning of a gene into another
virus
By cloning the gene for a protective antigen into different harmless virus, the antigen
is presented just as in the original virus. In addition, cells become infected, leading to
cell-mediated immunity. Vaccinia (the smallpox vaccine virus) is a good
candidate since it has been widely used in the human population with no ill
effects. Moreover, a multivalent vaccine virus strain can be made in this way as vaccinia will accept several
foreign genes. A candidate HIV vaccine has undergone clinical trials. However, the use of vaccinia against
smallpox has shown rare but serious complications in immuno-compromised patients
and alternatives have been sought. One is a recombinant canary pox virus that does not
replicate in humans but does infect cells. Immunization with live recombinant canary pox
vector expressing the HIV envelope gene has induced an HIV-1 envelope specific CTL response. Similar
constructs with gag, protease, nef and parts of pol genes have been studied in clinical trials
but all have, so far, shown no clinical efficacy.
Today the most widely used virus vector is a modified
human or chimpanzee adenovirus which have been used in several COVID-19
vaccines.
|
| |
DNA Vaccines
These vaccines are based on
the introduction of a DNA plasmid or whole virus into the vaccinee. The vaccine
carries an extra protein-coding
gene that expresses an antigen that causes an immune response; for example, some
COVID-19 vaccines use a replication-deficient adenovirus expressing the
SARS-CoV-2 S protein.
These vaccines are often called DNA vaccines but would better
be called DNA-mediated or DNA-based immunization since it is not the purpose to raise
antibodies against the DNA molecules themselves but to get the protein expressed
by cells of the vaccinee. Usually, muscle cells do this since the DNA is
injected intramuscularly.
|
|
WEB RESOURCES
DNA
vaccine web
|
| |
Advantages of DNA vaccines
-
Plasmids or DNA
viruses are easily manufactured in large amounts
-
DNA is very stable
-
DNA resists temperature extremes
and so storage and transport are
straight forward
-
A DNA sequence can be changed easily in the laboratory. This means
that we can respond rapidly to variants in the infectious agent. This has become
particularly important in developing vaccines against COVID-19.
-
By using DNA
injected into the vaccinee to code for antigen synthesis,
the antigenic protein(s) that are produced are processed (post-translationally modified)
in the same way as the proteins of the virus against which protection is to be produced.
This makes a far better antigen than, for example, using a recombinant
plasmid to produce an antigen in yeast (e.g. the HBV vaccine), purifying that protein and
using it as an immunogen.
-
Mixtures of DNA
constructs could be used that encode many protein
fragments from a virus or viruses so that a broad spectrum vaccine could be produced
-
The DNA construct does not replicate
in the vaccinee and encodes only the proteins of
interest
-
Because of the way the antigen is presented, there is a
cell-mediated response that may be directed against any antigen in the pathogen.
This also offers
protection against diseases caused by certain obligate intracellular pathogens (e.g.
Mycobacterium tuberculosis)
All of the above means that DNA vaccines are cheap and therefore likely
to be developed against pathogens of lesser economic importance (at least to drug
companies)
|
| |
Possible Problems
-
Potential integration of
DNA into the host genome leading to insertional mutagenesis
-
Induction of autoimmune responses (e.g. pathogenic anti-DNA
antibodies)
-
Induction of immunologic
tolerance (e.g. where the expression of the antigen in the host may lead to
specific non-responsiveness to that antigen)
|
| |
The current influenza vaccine is an inactivated preparation
containing antigens from the flu strains
that are predicted to infect during the next flu
season. If such a prediction goes awry, the vaccine is of little use. It is the surface
antigens that change as a result of reassortment of the virus in the animal (duck)
reservoir (see
influenza). The vaccine is injected intramuscularly and elicits an IgG response
(humoral
antibody in the circulation). The vaccine is protective because enough of the IgG gets
across the mucosa of the lungs where it can bind and neutralize incoming virus by binding
to surface antigens. If a DNA vaccine is used, both humoral and cytotoxic T
lymphocytes (CTL) are produced, which recognize antigens presented by vaccine-transfected
cells. The CTLs are produced because the infected muscle cells present flu antigens in association with MHC class I
molecules. If the antigen presented is the nucleocapsid protein (which is a conserved
protein), this overcomes the problem of antigenic variation. Such an approach could
revolutionize the influenza vaccine.
Other studies have used a mix of plasmids encoding both nucleoprotein
and surface antigens. Protection by DNA vaccines has also been demonstrated with rabies,
mycoplasma
and Plasmodium yoelii.
Anti-HIV vaccines are also being tested. In the
HIV chapters, it was noted that
progress on AIDS vaccines has been stymied by the fact that many vaccines only elicit
humoral antibodies while the use of whole virus vaccines (which might elicit CTL
responses) has been rejected because of other potential problems. Plasmid-based
vaccines may overcome these problems
ADENOVIRUS-BASED DNA VACCINES
AstraZenica/Oxford University
Vaccine: AZD1222, CHADOX1 NCOV-19
The ChAdOx1 nCoV-19 vaccine (AZD1222) consists of the
replication-deficient simian adenovirus vector ChAdOx1, containing
the full-length S glycoprotein gene of SARS-CoV-2, with a tissue
plasminogen activator leader sequence. ChAdOx1 nCoV-19 expresses a
codon-optimized coding sequence for the S protein. A simian
adenovirus rather than a human one is used because the use of human
adenovirus is limited by pre-existing immunity to the virus within
the human population that significantly reduces the immunogenicity
of vaccines based on the human virus. This is not a problem with the
simian virus because, although simian adenoviruses are closely
related to human adenoviruses, the hypervariable regions of the main
immunogen are significantly different from the human virus thus
avoiding preexisting immunity. The simian adenovirus vectors lack
the E1 region encoding viral transactivator proteins which are
essential for virus replication and the E3 region encoding
immunomodulatory proteins. The latter deletion allows incorporation
of larger genetic sequences into the viral vector. The vaccine
adenovirus is taken up by cells and is transcribed in the nucleus to
give mRNA which is translated to S protein. Efficacy is up to 90%,
depending on the dosage. Higher efficacy was found in a subgroup in
which the first of two doses was halved. The average efficacy was
70.4%.
AD5-NCOV, Convidicea (Cansino
Biologics, China) This is another adenovirus-based
vaccine. It is based on recombinant replication-defective human
adenovirus type-5 vector to induce an immune response. Again, the
virus has been rendered replication-deficient by deletion of the E1
and E3 genes. It encodes an optimized full-length S protein gene
based on Wuhan-Hu-1 virus sequence with the tissue plasminogen
activator signal peptide gene.
GAM-COVID-VAC, Sputnick V (Gamaleya
Research Institute of Epidemiology and Microbiology, Russia) Gam-COVID-Vac is a two-vector vaccine based on two modified human
adenoviruses containing the gene that encodes the S protein of
SARS-CoV-2. The first inoculation uses adenovirus 26 (Ad26) as the
vector for the S protein gene while the second uses adenovirus 5
(Ad5). This vaccine was shown in January, 2021, to have 91.6%
efficacy against symptomatic Covid-19. AD26.COV2.S, JNJ-78436735
(Janssen/Johnson and Johnson, United States and Belgium)
This vaccine is based again on a recombinant modified adenovirus
vector. Like the Sputnick vaccine, it uses human Ad26 expressing the
S protein, in this case in a single inoculation. It raises a strong
neutralizing antibody and cell-mediated response. It uses AdVac
technology which increases stability so that the vaccine may be
stored at refrigerator temperatures for at least three months.
MRNA VACCINES
The
first two vaccines for COVID-19 approved in late 2020 were based on a protocol in
which mRNA coding for the antigen of interest surrounded by a lipid
carrier (lipid nanoparticle) is injected into the vacinee. The lipid
protects the mRNA from ribonucleases and facilitates its entry into
cells. The mRNA is translated to protein, processed and presented to the
immune system in the usual way. The protein of interest is usually that
which binds to the cell receptor and antibodies to this protein which
block virus-cell receptor interaction will prevent infection and are
called neutralizing antibodies. In the case of vaccines against
SARS-CoV-2, this is the S antigen that binds to the human ACE2 receptor.
A major problem with mRNA vaccines is their stability in transit from
the site of manufacture, outside the cell at the site of injection and
within the cell. DNA Is inherently stable within the cell since it must
pass the genetic code from cell to cell indefinitely. In contrast, mRNAs
have a very short life compared to DNA. The amount of a mRNA depends on
the balance between the rate of synthesis and the rate of degradation.
Many proteins are required only for a very short time, and if their
mRNAs were very stable the protein level could not be controlled. Hence,
although all mRNAs have short lives, many are degraded very rapidly
after translation, facilitating rapid responses to the conditions in the
cell. The mRNAs are degraded by ribonucleases (RNAses). Different mRNAs
have different degrees of stability resulting from their secondary
structure and the nature of the ends of molecule. These are known as cis
elements. In addition, their stability is also regulated by RNA-binding
factors or trans elements. Cis elements include the 3’ poly A tail and
the 5’ methyl guanosine cap. The 3’ poly A tail is bound by poly
A-binding proteins that stabilize the RNA. These proteins require a
certain length of poly A tail to bind and so the longer the poly A tail,
the more of these proteins can bind to the RNA. mRNA is degraded from
the 3’ end by 3’-5’ exonucleases and the 5’ end by removal of the 5’ cap
and 5’-3’ exonuclease activity. Endonuclease activity also degrades mRNA
and this can be regulated by other RNA binding proteins. AU-rich
sequences in the 3’ untranslated region (UTR) are also involved in
stability.
MRNA may also be stabilized by chemical modification of the bases of
the nucleic acid itself. Such modifications include methyl adenosine,
N-1-methylpseudouridine and pseudouridine (made from uridine by
pseudouridine synthase (figure 13)), a base modification that is common
in tRNA and enhances its stability. In mRNA these substituted bases
enhance translation. Pseudouridine and N-1-methylpseudouridine repress
intracellular signaling triggers for protein kinase R activation which
is involved in mRNA stability. Of course, such modifications must not
alter the fidelity of the translation of the message. MRNA vaccines
are made by the transcription of a plasmid encoding a protein recognized
by a neutralizing antibody, in the case of a Covid-19 vaccine, this is
the S protein. The plasmid, which contains the appropriate promoter
sequences, is linearized and transcribed in vitro using a T7, T3 or Sp6
phage RNA polymerase. The resulting product contains an open reading
frame that encodes the S protein flanked by 5’ and 3’ UTRs, a 5’ methyl
guanosine cap and a poly A tail. This is what is used as the vaccine.
Figure 14 shows one way that this might be done in a system from AmpTec.
The S protein gene is cloned into an insertion site in an m13 plasmid
along with a T7 promotor (A). A forward primer complementary to the end
of the M13 sequence (Pri) and a second reverse primer complementary to
the end of the S gene are used (B). The latter primer includes a poly T
sequence, usually around 120 nucleotides which does not hybridize to any
m13 sequence. Using PCR, the DNA structure shown in C is produced. This
is then used in in vitro transcription from the T7 promoter to form the
polyadenylated mRNA shown in D. In vitro transcription can be carried
out in the presence of modified nucleotides such as pseudouracil and/or
N6-methyl adenosine, 5-methyl cytidine and others. These modified mRNAs
are much more stable than normal mRNAs and are highly translatable
giving the vaccine much increased efficacy.
The resultant protein is processed in the normal way through the
exocytic pathway with all the usual post-translational modifications
including glycosylation and transported to the cell surface. As
described above, the protein may also be cleaved by proteases to form
small peptides that can be presented at the cell surface to the immune
system. The cell has anti-viral mechanisms to detect and degrade foreign
RNAs and steps are taken to minimize this.
Even with nucleotide modifications, naked mRNA is likely to be
rapidly degraded when injected into the vaccinee. In addition, the mRNA
must cross the cell membrane to gain access to the cell protein
translation machinery. Both of these problems can be solved by
encapsulating the mRNA in a lipid envelope (a lipid nanoparticle or
liposome) that helps the mRNA vaccine enter the cytoplasm from the
endosome before it is degraded in a lysosome.
The initial Covid mRNA vaccines from BioNtec and Moderna use a
technology similar to the above. A modification that may well be used in
future mRNA vaccines is to make an mRNA vaccine which contains not only
mRNA for the protein of choice (e.g. the SARS-CoV-2 S protein) but also
mRNA for a viral RNA-dependent RNA polymerase (replicase). When this
type of mRNA vaccine is injected into a vaccinee and enters a cell, it
will be translated into S protein and into the replicase (which may be
encoded on the same mRNA or a second mRNA). The viral replicase can
recognize viral replication signals included in the vaccine mRNA(s) and
can then amplify the input vaccine mRNA, making more copies of the mRNA
and therefore more of the protein. Since there is now more of the
vaccine mRNA in the cell than was originally delivered to the cytoplasm,
this is known as the self-amplifying (SA) mRNA approach.
Tozinameran (BNT162B2.
Brand name: Comirnaty) Pfizer-BioNTech Covid-19 vaccine
Tozinameran was the first mRNA vaccine to be approved. In clinical
trials its efficacy is around 95%, 28 days after the first dose and
is well tolerated. In one of the initial trials, there were 170
confirmed cases of Covid-19 of which 162 were in the placebo group
and only 8 in the vaccine group. It is given in two doses, three
weeks apart. It was not evaluated for asymptomatic infection. It
appears to be effective against the variants described above. This
vaccine must be stored and transported at -70 C. It contains (WHO
Non-proprietary names Program):
A modified 5’-cap1 structure (m7G+m3'-5'-ppp-5'-Am)
5´-untranslated region derived from human alpha-globin RNA with
an optimized Kozak sequence. The latter ensures that the protein
is correctly translated by the ribosome and functions as the
translation initiation site in most eukaryotic mRNAs.
S glycoprotein signal peptide necessary for directing the
nascent protein/ribosome complex to the signal receptor on the
cytoplasmic surface of the rough endoplasmic reticulum membrane.
This guides protein translocation to the correct orientation in
the endoplasmic reticulum.
Codon-optimized sequence encoding full-length SARS-CoV2 S
protein that contains two mutations: K986P and V987P. These
alter the folding of the S protein so that it adopts an
antigenically optimal pre-fusion conformation. All of the
uridines are replaced by 1- methyl-3’-pseudouridine residues (Ψ)
(figure 15) that are nevertheless efficiently translated.
At the end of the coding sequence are two ΨGA stop codons
The 3´ untranslated region comprises two sequence elements that
confer RNA stability and high protein expression.
A 110-nucleotide poly A-tail consisting of a stretch of 30
adenosine residues, followed by a 10-nucleotide linker sequence
and another 70 adenosine residues.
In addition the vaccine contains lipids that make up the solid
lipid nanoparticles that encapsulate the mRNA (ALC-0315 =
((4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate);
ALC-0159 = 2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide;
1,2-Distearoyl-sn-glycero-3-phosphocholine; and cholesterol. In
addition, the vaccine contains water, sucrose, dibasic sodium
phosphate dehydrate, monobasic potassium phosphate, potassium
chloride and sodium chloride.
Moderna Vaccine. mRNA1273
The Moderna vaccine is also an mRNA consisting of a synthetic
message encoding the pre-fusion stabilized spike glycoprotein of
SARS-CoV-2 virus. Pre-fusion stabilization is achieved by the
substitution of two prolines as in the BioNTech vaccine. Again,
the mRNA is made by transcription from a T7 promotor in a reaction
in which UTP was substituted with 1-methylpseudoUTP. In addition to
the mRNA, the vaccine contains lipids to form a lipid nanoparticle:
(SM-102, 1,2-dimyristoyl-rac-glycero3-methoxypolyethylene
glycol-2000 [PEG2000-DMG], cholesterol, and
1,2-distearoyl-snglycero-3-phosphocholine) and, tromethamine,
tromethamine hydrochloride, acetic acid, sodium acetate, sucrose and
water. The efficacy of m1273 is around 94.1%, similar to the BioNTec vaccine.
In an initial trial, there were 196 confirmed cases of Covid-19 of
which 185 were in the placebo group and 11 in the vaccine group. It has the advantage over the latter in that the
different lipid nanoparticle formulation allows it to be stored and
transported at 2-8C, rather than the -70C of the BioNTec vaccine. It
is administered in two doses, three weeks apart.
OTHER COVID-19 VACCINES
In the race to develop vaccines for COVID-19, many older strategies that have
shown success in the past have also been used. These include subunit vaccines,
an approach similar to that used to develop the highly successful vaccines for
hepatitis B, and inactivated virus particles, an approach first used for the
Salk polio vaccine.
Subunit vaccine: NVX-CoV2373, Novavax
The Novavax vaccine (NVX-CoV2373) is based on older technology using
purified SARS-CoV-2 S protein with a Matrix M adjuvant.
In clinical trials, it produced high levels of anti-S protein
antibodies and has been ordered by several governments as part of
their anti-Covid-19 strategy. The gene for the S protein is inserted
into a baculovirus. The Baculoviridae are a family of
double-stranded circular DNA (80-180 base pairs) viruses that infect
insects and arthropods. The modified baculovirus is then use to
infect insect cells (usually Sf9 cells, isolated from Spodoptera
frugiperda, the fall army worm) which make the S protein. This
assembles into native trimers on the surface of the infected cell.
These proteins are extracted and associated with lipid nanoparticles
so that the S protein is presented to the immune system in a manner
similar to that on the surface of an infected cell. Included with
the vaccine is an adjuvant extracted from Quillaja saponaria,
the soap bark tree (which, as its name implies can be used as a
soap). In the case of vaccines, it stimulates the attraction of
immune cells to the site of the injection where they respond more
effectively. The adjuvant properties come from saponins (triterpene
glycosides). The nanoparticles containing the S protein are taken up
by antigen-presenting cells, cleaved into peptides and presented on
the cell surface in association with MHC antigens to T and B cells. Phase 3 trials have shown that this vaccine has 89% efficacy against
Covid-19 and appears to provide strong immunity against the UK and
South African variants.
Inactivated virus particles:
Valneva vaccine (VLA 2001)
This uses an even more established vaccine technology similar to
that used in the Salk polio vaccine, that is the use of inactivated
whole virus particles. Virus is grown on African Green Money Kidney
(Vero) cells, purified and inactivated with an agent such as
formalin. The vaccine also contains alum and CpG 1018 adjuvants. CpG
1018 is a toll-like receptor 9 (TLR9) agonist. There are a number of other SARS-CoV-2 vaccines in phase I and II
trials that use formalin-inactivated
whole virus particles (Sinovac and Sinopharm)
WHY DO WE NEED TWO
INOCULATIONS? Most of the vaccines that have been developed
against SARS-CoV-2 require two inoculations. This is because of the way
that the immune system responds to a foreign pathogen such as an
infecting virus.
Initially, it is important to suppress infection by stopping the
invading pathogen entering cells and replicating. Infection by a virus
binding to its receptor on the cell surface (ACE2 in the case of
SARS-CoV-2) triggers an initial response in which plasma B lymphocytes
produce neutralizing antibodies that bind to the surface of the invading
organism thereby, in the case of SARS-CoV-2, blocking virus S protein
binding to ACE2. The initial antibody response, however, quickly
declines but some of the B cells differentiate into memory B cells that
survive for a long time and relocate to the periphery of the body. Here,
they will be more likely to encounter more antigen during a second
exposure. When this happens, they proliferate and differentiate into
more plasma B cells, which then respond to the antigen by producing more
antibodies. Memory B cells can survive for many years so that they are
able to respond to multiple exposures to the same antigen. During the
first phase of the immune response, the immune cells also secrete
cytokines that recruit other immune cells to the site of infection,
among which are CD4-positive helper and cytotoxic T cells (killer T
cells) that recognize and kill virus-infected cells. As with B cells,
some T cells differentiate into memory cells that can reactivate and
proliferate in response to new exposure to the original antigen. These
memory T cells can also remain in the body for many years (and perhaps
for a lifetime). Since only a small number of memory T cells are made
as a result of the initial exposure, a second exposure to the antigen
(infection or inoculation) is required to boost their levels. Thus, with
the mRNA SARS-CoV-2 vaccines, protection starts about 12 days after the
first inoculation and rises to about 50% effectiveness. After a second
injection three to four weeks later, the second phase of the immune
response starts, memory B and T cells increase and effectiveness rises
to around 95%.
|
|
 Figure 13
Figure 13
Pseudouridine and uridine structure
 Figure 14
Figure 14
Transcription of an mRNA vaccine molecule from a DNA plasmid construct
 Figure 15
Figure 15
1-methylpseudouridine. An extra methyl group is added enzymatically to
the base of the pseudouracil
|
 Figure 13
Vaccine-preventable diseases, by year of vaccine development or
licensure - United States, 1798-1998 (MMWR/CDC)
Figure 13
Vaccine-preventable diseases, by year of vaccine development or
licensure - United States, 1798-1998 (MMWR/CDC) |
Today, many anti-viral vaccines are available and more are being
developed. These vaccines have made a considerable impact on public
health around the world (figure 13 and and see
here).
|
|
WEB
RESOURCES
Baseline
20th century annual morbidity and 1998 provisional morbidity from nine
diseases with vaccines recommended before 1990 for universal use in
children in the United States
MMWR/CDC
Are your
child's vaccines up to date?
CDC |
| |
 Return to the Virology section of Microbiology and Immunology On-line
Return to the Virology section of Microbiology and Immunology On-line
This page last changed on
Monday, February 15, 2021
Page maintained by
Richard Hunt
|

 Figure 1b
Figure 1b Figure 4.
Figure 4. Figure 8
Figure 8 Figure 10a
Figure 10a Figure 11 Attenuated influenza vaccine strain using a cold-sensitive mutant that
can be reassorted with new virulent strains
Figure 11 Attenuated influenza vaccine strain using a cold-sensitive mutant that
can be reassorted with new virulent strains
 Figure 12 Anti-idiotype antibodies
Figure 12 Anti-idiotype antibodies  Figure 13
Vaccine-preventable diseases, by year of vaccine development or
licensure - United States, 1798-1998 (MMWR/CDC)
Figure 13
Vaccine-preventable diseases, by year of vaccine development or
licensure - United States, 1798-1998 (MMWR/CDC)








































